Most of the solvents with abbreviated names are polar aprotic 64 Elimination Reactions Higher temperatures increase the rates of elimination reactions A product with a more substituted double bond is more stable and thus more favorable If tert-butoxide is used sterics must be considered to nd out which hydrogen it takes through the E2 reaction. The hydrogen-fluorine bond is so strong that fluorine radicals arent formed in the initiation step.
 Stereoselectivity Of E2 Elimination Reactions Practice Problems Reactions Organic Chemistry Chemistry
Stereoselectivity Of E2 Elimination Reactions Practice Problems Reactions Organic Chemistry Chemistry
Lets look at the mechanism for this reaction.

Draw the most stable product formed in each of the following reactions. Substitution Reactions With Hydride Shifts In this post we cover several examples of reactions where carbocations form but then a funny thing happens. A C-H bond interacts with the empty p-orbital and before you know it the C-H bond has moved and a new more stable. Draw an arrow pushing mechanism for the acid catalyzed dehydration of the following alcohol make sure to draw both potential mechanisms.
Draw the mechanism of its formation. Here are three examples that will help you think through the main issues answers in the next post. Benzene Derivatives Substitution Reactions of Benzene and Other Aromatic Compounds.
Unlike E2 reactions E1 is not stereospecific. Addition of HBr to 23-dimethyl-13-cyclohexadiene may occur in the absence or presence of peroxides. This is a HUGE question to answer completely.
In each case two isomeric C 8 H 13 Br products are obtained. Question from very important topics are covered by NCERT Exemplar Class 11You also get idea about the type of questions and method to answer in your Class 11th examination. One answer is because they result in a negative change in free energy delta-G This may be as a result of the reaction being exothermic so the products are more stable than the reactants or could be a result of an increase in entropy products more disordered than the reactants or both of these.
I Acetaldehyde CH3CHO reacts with hydrogen cyanide HCN to give 2-Hydroxypropapanenitrile as product. Hint a rearrangement occurs 9. Which of the following will be the kinetically favored product from the depicted reaction.
NCERT Exemplar Class 11 Chemistry is very important resource for students preparing for XI Board Examination. Ii Acetaldehyde CH3CHO reacts with Hydroxylamine NH2OH to give acetaldoxime as a product. Most simple alkyl amines have pK a s in the range 95 to 110 and their water solutions are basic have a pH of 11 to 12 depending on concentration.
Draw the product of the following reaction 2513c Draw the product of the following reaction. 14 pt Draw the organic product expected from each of the following reactions. The last five compounds colored cells are significantly weaker bases as a consequence of three.
In many cases one major product will be formed the most stable alkene. Draw the most stable chair conformation for each of the following 1. With the other hydrogen halides the opposite is true.
In the case of butadiene it is true that the 12 product was formed through a more stable carbocation kinetic product and the 14 product had a more stable double bond thermodynamic product. Here we have provided NCERT Exemplar Problems Solutions along with NCERT Exemplar Problems Class 11. CH3 H Br D NaOCH3 CH3OH.
Iii The reaction of acetaldehyde with acetaldehyde in the presence of dilute NaOH this is the kind of Aldol reaction by which obtained 3-hydroxybutanal as a product. Be sure to indicate stereochemistry where appropriate and to include stereoisomers if any. Thus a hydrogen is not required to be anti-periplanar to the leaving group.
The remarkable stability of the unsaturated hydrocarbon benzene has been discussed in an earlier chapterThe chemical reactivity of benzene contrasts with that of the alkenes in that substitution reactions occur in preference to addition reactions as illustrated in the following diagram some comparable. Elimination Reactions and Alkene Synthesis 1 One of the products that results when 1-bromo-22-dimethylcyclopentane is heated in ethanol is shown below. Give a mechanism by which it is formed and give the name of this mechanism.
But it will not always be true for all dienes. An adjacent bonding pair of electrons ie. Both mechanisms happen but most of the product is the one from the free radical mechanism because that is working faster.
In double displacement reactions or metathesis reactions two species normally ions are displaced. In the dehydration of this diol the resulting product is a ketone. Which of the following is a common product from both reactions.
In case two or more stereoisomers are formed label their relationship as diastereomers enantiomers structural isomers or conformers. Assume no rearrangement for the first two product mechanisms. The first four compounds in the following table including ammonia fall into that category.
In terms of regiochemistry Zaitsevs rule states that when more than one product can be formed the more substituted alkene is the major product. CH3 CH3 2 Provide the structure of the major organic product in the following reaction.
 E1 Energy Diagram Transition State Forming A Double Bond Reactions Energy Activities Chemistry
E1 Energy Diagram Transition State Forming A Double Bond Reactions Energy Activities Chemistry
 Organic Chemistry 5th Edition Textbook Solutions Chegg Com Crossed Aldol Reaction Organic Chemistry Chemistry Textbook
Organic Chemistry 5th Edition Textbook Solutions Chegg Com Crossed Aldol Reaction Organic Chemistry Chemistry Textbook
 The Mechanisms Of E2 Elimination And Sn2 Nucleophilic Substitution Reactions Reactions Hydrogen Bond Organic Chemistry
The Mechanisms Of E2 Elimination And Sn2 Nucleophilic Substitution Reactions Reactions Hydrogen Bond Organic Chemistry
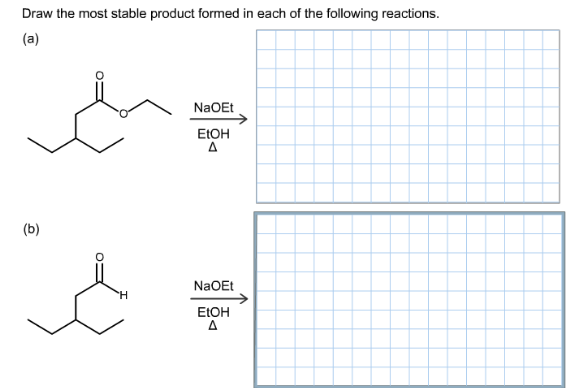 Draw The Most Stable Product Formed In Each Of The Following Reactions Home Work Help Learn Cbse Forum
Draw The Most Stable Product Formed In Each Of The Following Reactions Home Work Help Learn Cbse Forum
 E1 Elimination Mechanism Kinetcis Practice Problems Organic Chemistry Chemistry Reactions
E1 Elimination Mechanism Kinetcis Practice Problems Organic Chemistry Chemistry Reactions
 Label The Beta Hydrogens And Using Curved Arrows Draw The Mechanism For The Following Elimination Reactions Assuming A Co Chemistry Reactions Organic Chemistry
Label The Beta Hydrogens And Using Curved Arrows Draw The Mechanism For The Following Elimination Reactions Assuming A Co Chemistry Reactions Organic Chemistry
 How To Quickly Predict The Product Of An Aldol Reaction Chemistry Organic Chemistry Study Organic Chemistry
How To Quickly Predict The Product Of An Aldol Reaction Chemistry Organic Chemistry Study Organic Chemistry
 E1 Reactions Are Stereoselective E Cis Stereoisomer Is The Major Product Chemistry Lecture Chemistry Reactions
E1 Reactions Are Stereoselective E Cis Stereoisomer Is The Major Product Chemistry Lecture Chemistry Reactions
 Syn Dihydroxylation Of Alkenes With Kmno4 And Oso4 Practice Problems Chemistry Chemistry Lecture Organic Chemistry
Syn Dihydroxylation Of Alkenes With Kmno4 And Oso4 Practice Problems Chemistry Chemistry Lecture Organic Chemistry
 Vsepr Model Google Search Vsepr Theory Chemistry Theories
Vsepr Model Google Search Vsepr Theory Chemistry Theories
 Nucleophilic Aromatic Substitution Practice Predict The Product Organic Chemistry Study Chemistry Chemistry Lecture
Nucleophilic Aromatic Substitution Practice Predict The Product Organic Chemistry Study Chemistry Chemistry Lecture
 E2 Stereoselectivity Shown In Newman Projections Chemistry Notes Organic Chemistry Chemistry
E2 Stereoselectivity Shown In Newman Projections Chemistry Notes Organic Chemistry Chemistry
 Determine If Each Of The Following Alkenes And Cycloalkanes Has An E Or Z Configuration Chemistry Mcat Study Chemistry Lecture
Determine If Each Of The Following Alkenes And Cycloalkanes Has An E Or Z Configuration Chemistry Mcat Study Chemistry Lecture
 Stereoselectivity Of E1 Reactions Practice Problems Chemistry Worksheets Reactions Chemistry
Stereoselectivity Of E1 Reactions Practice Problems Chemistry Worksheets Reactions Chemistry
Https Encrypted Tbn0 Gstatic Com Images Q Tbn And9gcq3q1e0avnpbrjdsgqet7ggb8et5fdd3kpia Hbcfrpkptfd4z2 Usqp Cau
 E1 Dehydration Of Alcohols Practice Problems Predict The Product When Heated With H2so4 Biologie Wiskunde Rekenvaardigheid
E1 Dehydration Of Alcohols Practice Problems Predict The Product When Heated With H2so4 Biologie Wiskunde Rekenvaardigheid
 The E1 And Sn1 Reactions Always Compete Since Both Form The Same Carbocation Reactions How To Remove Practice
The E1 And Sn1 Reactions Always Compete Since Both Form The Same Carbocation Reactions How To Remove Practice
 Electrophilic Aromatic Substitution Directing Groups Master Organic Chemistry Organic Chemistry Chemistry Basics Chemistry
Electrophilic Aromatic Substitution Directing Groups Master Organic Chemistry Organic Chemistry Chemistry Basics Chemistry
 Rearrangements 1 2 Hydride Shift Summary Chemistry Chemistry Lecture Reactions
Rearrangements 1 2 Hydride Shift Summary Chemistry Chemistry Lecture Reactions
 Sn1 Mechanism The Nucleophile Attacks From Both Sides Carbocation Giving A Racemic Mixture Chemistry Lessons Chemistry Worksheets Chemistry Lecture
Sn1 Mechanism The Nucleophile Attacks From Both Sides Carbocation Giving A Racemic Mixture Chemistry Lessons Chemistry Worksheets Chemistry Lecture
 E And Z Configuration Of Double Bonds Based On Group Priority Chemistry Configuration Organic Chemistry
E And Z Configuration Of Double Bonds Based On Group Priority Chemistry Configuration Organic Chemistry
 Zaitsev S Rule Regioselectivity Of E2 Elimination Reactions Practice Problems Chemistry Lessons Reactions Organic Synthesis
Zaitsev S Rule Regioselectivity Of E2 Elimination Reactions Practice Problems Chemistry Lessons Reactions Organic Synthesis
 Electrophilic Aromatic Substitution Directing Groups Master Organic Chemistry Organic Chemistry Chemistry Basics Chemistry
Electrophilic Aromatic Substitution Directing Groups Master Organic Chemistry Organic Chemistry Chemistry Basics Chemistry
 Eas Reactions 3 Friedel Crafts Acylation And Friedel Crafts Alkylation Organic Chemistry Chemistry Organic Chem
Eas Reactions 3 Friedel Crafts Acylation And Friedel Crafts Alkylation Organic Chemistry Chemistry Organic Chem
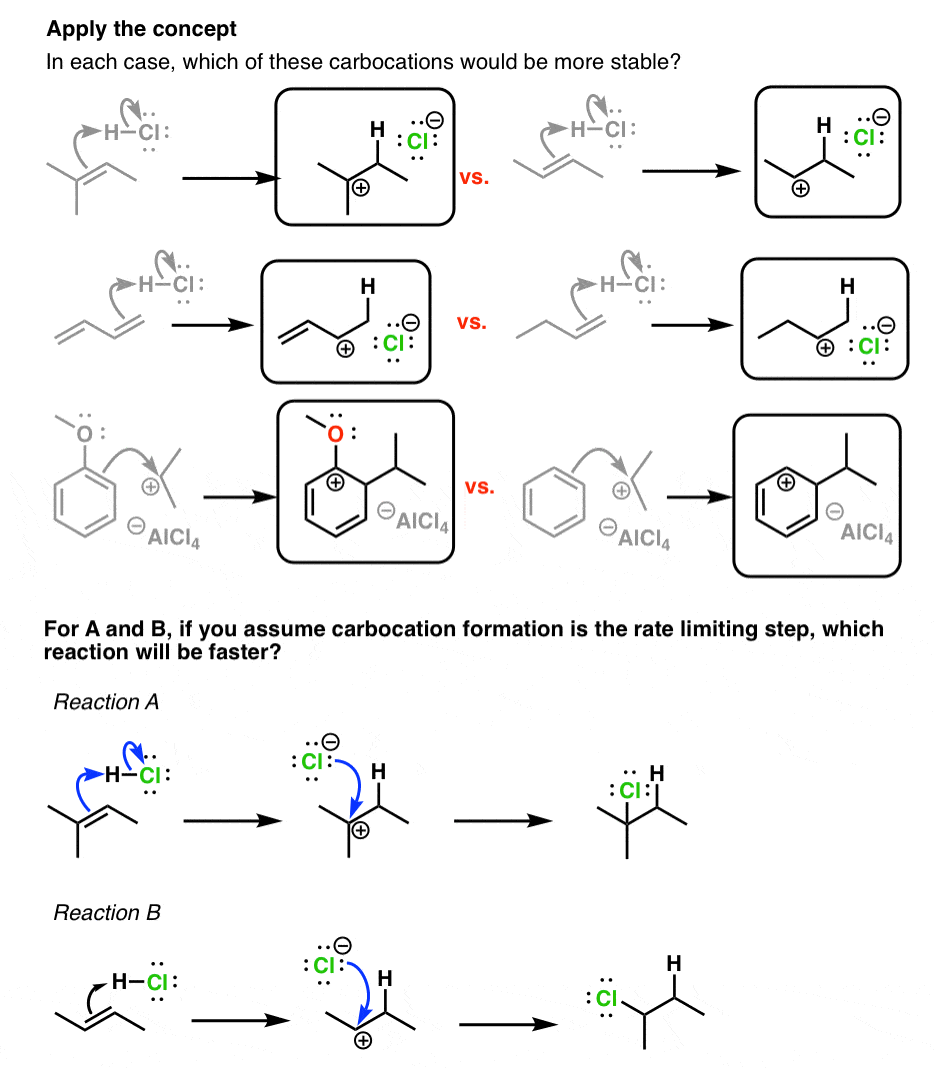 3 Factors That Stabilize Carbocations
3 Factors That Stabilize Carbocations
 Carbocation Rearrangements In Sn1 Reactions Practice Problems Organic Chemistry Reactions Organic Chemistry Study Chemistry
Carbocation Rearrangements In Sn1 Reactions Practice Problems Organic Chemistry Reactions Organic Chemistry Study Chemistry