draw the correct product for the following diels alder reaction
Nicolaou, K. C. & Montagnon, T. Molecules that Changed the World: A Brief History of the Art and Science of Amalgam and its Impact on Society (Wiley-VCH, 2008).
["628.56"]Nicolaou, K. C. & Sorensen, E. J. Classics in Absolute Amalgam (Wiley-VCH, 1995).
Nicolaou, K. C. & Snyder, S. A. Classics in Absolute Amalgam II (Wiley-VCH, 2003).
Walji, A. M. & MacMillan, D. W. C. Strategies to bypass the Taxol problem. Enantioselective avalanche catalysis, a new admission for the able architecture of atomic complexity. Synlett 1477–1489 (2007).
Staunton, J. & Weissman, K. J. Polyketide biosynthesis: a millennium review. Nature Prod. Rep. 18, 380–416 (2001).
Townsend, C. A. Structural studies of accustomed artefact biosynthetic proteins. Chem. Biol. 4, 721–730 (1997).
Floss, H. G. & Yu. T.-W. Rifamycin-mode of action, resistance, and biosynthesis. Chem. Rev. 105, 621–632 (2005).
Davies, H. M. L. & Sorensen, E. J. Rapid complication bearing in accustomed artefact absolute synthesis. Chem. Soc. Rev. 38, 2981–2982 (2009).
Nicolaou, K. C. & Chen, J. S. The art of absolute amalgam through avalanche reactions. Chem. Soc. Rev. 38, 2993–3009 (2009).
Nicolaou, K. C., Edmonds, D. J. & Bulger, P. G. Avalanche reactions in absolute synthesis. Angew. Chem. Int. Ed. 45, 7134–7186 (2006).
Young, I. S. & Baran, P. S. Protecting-group-free amalgam as an befalling for invention. Nature Chem. 1, 193–205 (2009).
Burns, N. Z., Baran, P. S. & Hoffmann, R. W. Redox abridgement in amoebic synthesis. Angew. Chem. Int. Ed. 48, 2854–2867 (2009).
Trost, B. M. The atom abridgement − a chase for constructed efficiency. Science 254, 1471–1477 (1991).
Wender, P. A., Verma, V. A., Paxton, T. J. & Pillow, T. H. Function-oriented synthesis, footfall economy, and biologic design. Acc. Chem. Res. 41, 40–49 (2008).
Kim, J. & Movassaghi, M. Biogenetically aggressive syntheses of alkaloid accustomed products. Chem. Soc. Rev. 38, 3035–3050 (2009).
Bulger, P. G., Bagal, S. K. & Marquez, R. Recent advances in biomimetic accustomed artefact synthesis. Nat. Prod. Rep. 25, 254–297 (2008).
Newhouse, T., Baran, P. S. & Hoffmann, R. W. The economies of synthesis. Chem. Soc. Rev. 38, 3010–3021 (2009).
Tietze, L. F., Brasche, G. & Gericke, K. M. Domino Reactions in Amoebic Amalgam (Wiley-VCH, 2006).
Enders, D., Grondal, C. & Hüttl, M. R. M. Agee organocatalytic domino reactions. Angew. Chem. Int. Ed. 46, 1570–1581 (2007).
Walji, A. M. & MacMillan, D. W. C. Strategies to bypass the taxol problem. Enantioselective avalanche catalysis, a new admission for the able architecture of atomic complexity. Synlett 1477–1489 (2007).
Bertelsen, S. & Jørgensen, K. A. Organocatalysis - afterwards the gold rush. Chem. Soc. Rev. 38, 2178–2189 (2009).
Dondoni, A. & Massi, A. Agee organocatalysis: from boyhood to adolescence. Angew. Chem. Int. Ed. 47, 4638–4660 (2008).
["1237.72"]MacMillan, D. W. C. The appearance and development of organocatalysis. Nature 455, 304–308 (2008).
Dalko, P. I. Enantioselective Organocatalysis, Reactions and Experimental Procedures (Wiley-VCH, 2007).
Organocatalysis. Chem. Rev. 107 (special issue), 5413–5883 (2007).
de Figueiredo, R. M. & Christmann, M. Organocatalytic amalgam of drugs and bioactive accustomed products. Eur. J. Org. Chem. 2575–2600 (2007).
Berkessel, A. & Gröger, H. Agee Organocatalysis (Wiley-VCH, 2005).
Zhang, F.-L., Xu, A.-W., Gong, Y.-F., Wei, M.-H. & Yang, X.-L. Agee organocatalytic four basic quadruple domino acknowledgment accomplished by oxa-Michael accession of alcohols to acrolein. Chem. Eur. J. 15, 6815–6818 (2009).
Kotame, P., Hong, B.-C. & Liao, J.-H. Enantioselective amalgam of the tetrahydro-6H-benzo[c]chromenes via domino Michael–aldol condensation: ascendancy of bristles stereocenters in a quadruple-cascade organocatalytic multi-component reaction. Tetrahedron Lett. 50, 704–707 (2009).
Enders, D., Krüll, R. & Bettray, W. Microwave-assisted organocatalytic quadruple domino reactions of acetaldehyde and nitroalkenes. Amalgam doi:10.1055/s-0029-1217146 (2010).
Akiyama, T., Itoh, J. & Fuchibe, K. Recent advance in chiral Brønsted acerbic catalysis. Adv. Synth. Catal. 348, 999–1010 (2006).
Taylor, M. S. & Jacobsen, E. N. Agee catalysis by chiral hydrogen-bond donors. Angew. Chem. Int. Ed. 45, 1520–1543 (2006).
Enders, D., Niemeier, O. & Henseler, A. Organocatalysis by N-heterocyclic carbenes. Chem. Rev. 107, 5606–5655 (2007).
Lathrop, S. P. & Rovis, T. Agee amalgam of functionalized cyclopentanones via a multicatalytic accessory amine/N-heterocyclic carbene catalyzed avalanche sequence. J. Am. Chem. Soc. 131, 13628–13630 (2009).
Sun, F.-G., Huang, X.-L. & Ye, S. Diastereoselective amalgam of 4-hydroxytetralones via a avalanche Stetter−aldol acknowledgment catalyzed by N-heterocyclic carbenes. J. Org. Chem. 75, 273–276 (2010).
Sánchez-Larios, E. & Gravel, M. Diastereoselective amalgam of indanes via a domino Stetter−Michael reaction. J. Org. Chem. 74, 7536–7539 (2009).
Seebach, D. Methods of acuteness umpolung. Angew. Chem. Int. Ed. Engl. 18, 239–258 (1979).
Kaneko, S., Yoshino, T., Katoh, T. & Terashima, S. Constructed studies of Huperzine A and its fluorinated analogues. 1. Atypical agee syntheses of an enantiomeric brace of Huperzine A. Tetrahedron 54, 5471–5484 (1998).
Bai, D. Development of huperzine A and B for analysis of Alzheimer's disease. Authentic Appl. Chem. 79, 469–479 (2007).
List, B. The ying and yang of agee aminocatalysis. Chem. Commun. 819–824 (2006).
Yua, X. & Wang, W. Organocatalysis: agee avalanche reactions catalysed by chiral accessory amines. Org. Biomol. Chem. 6, 2037–2046 (2008).
Melchiorre, P., Marigo, M., Carlone, A. & Bartoli, G. Agee aminocatalysis - Gold blitz in amoebic chemistry. Angew. Chem. Int. Ed. 47, 6138–6171 (2008).
Marigo, M., Franzén, J., Poulsen, T. B., Zhuang, W. & Jørgensen, K. A. Agee organocatalytic epoxidation of α,β-unsaturated aldehydes with hydrogen peroxide. J. Am. Chem. Soc. 127, 6964–6965 (2005).
["564.54"]Yang, J. W., Hechavarria Fonseca, M. Y. & List, B. Catalytic agee reductive Michael cyclization. J. Am. Chem. Soc. 127, 15036–15037 (2005).
Huang, Y., Walji, A. M., Larsen, C. H. & MacMillan, D. W. C. Enantioselective organo-cascade catalysis. J. Am. Chem. Soc. 127, 15051–15053 (2005).
Bräse, S., Encinas, A., Keck, J. & Nising, C. F. Allure and analysis of mycotoxins and accompanying fungal metabolites. Chem. Rev. 109, 3903–3990 (2009).
Nising, C. F., Ohnemüller, U. K. & Bräse, S. The absolute amalgam of the fungal metabolite diversonol. Angew. Chem. Int. Ed. 45, 307–309 (2005).
Gérard, E. M. C. & Bräse, S. Modular syntheses of diversonol-type tetrahydroxanthone mycotoxins: blennolide C (epi-hemirugulotrosin A) and analogues. Chem. Eur. J. 14, 8086–8089 (2008).
Ohnemüller, U. K., Nising, C. F., Encinas, A. & Bräse, S. A able admission to enantiomerically authentic 5-substitued 4-hydroxycyclohex-2-enones: An avant-garde hemisecalonic acerbic A model. Amalgam 2175–2185 (2007).
Lesch, B. & Bräse, S. A short, atom-economical access to tetrahydroxanthenones. Angew. Chem. Int. Ed. 43, 115–118 (2003).
Stork, G. & Schultz, A. G. The absolute amalgam of dl-Camptothecin. J. Am. Chem. Soc. 93, 4074–4075 (1971).
Li, Q.-Y., Zu, Y.-G., Shi, R.-Z. & Yao, L.-P. Analysis camptothecin: accepted perspectives. Curr. Med. Chem. 13, 2021–2039 (2006).
Liu, G.-S., Dong, Q.-L., Yao, Y.-S. & Yao, Z.-J. Expeditious absolute syntheses of camptothecin and 10-hydroxycamptothecin. Org. Lett. 10, 5393–5396 (2008).
Dharmarajan, S., Perumal, Y., Rathinasabapathy, T. & Tanushree, R. B. Camptothecin and its analogues: a analysis on their chemotherapeutic potential. Nat. Prod. Res. 19, 393–412 (2005).
Yoshitomi, Y., Arai, H., Makino, K. & Hamada, Y. Enantioselective amalgam of martinelline chiral amount and its diastereomer appliance agee bike Michael–aldol reaction. Tetrahedron 64, 11568–11579 (2008).
Witherup, K. M. et al. Martinelline and martinellic acid, atypical G-protein affiliated receptor antagonists from the close bulb Martinella iquitosensis (bignoniaceae). J. Am. Chem. Soc. 117, 6682–6685 (1995).
Itoh, T., Yokoya, M., Miyauchi, K., Nagata, K. & Ohsawa, A. Absolute amalgam of ent-dihydrocorynantheol by appliance a proline-catalyzed agee accession reaction. Org. Lett. 8, 1533–1535 (2006).
Ibrahem, I., Sundén, H., Rios, R., Zhao, G.-L. & Córdova, A. One-pot pyrrolidine-catalyzed amalgam of benzopyrans, benzothiopyranes, and dihydroquinolidines. Chimia 61, 219–223 (2007).
Bertelsen, S., Marigo, M., Brandes, S., Dinér, P. & Jørgensen, K. A. Dienamine catalysis: organocatalytic agee γ-amination of α, β-unsaturated aldehydes. J. Am. Chem. Soc. 128, 12973–12980 (2006).
Liu, K., Chougnet, A. & Woggon, W.-D. A abbreviate avenue to α-tocopherol. Angew. Chem. Int. Ed. 47, 5827–5829 (2008).
Volz, N., Bröhmer, M. C., Nieger, M. & Bräse, S. Where are they now? An agee organocatalytic arrangement appear 4a-methyl tetrahydroxanthones: academic amalgam of 4-dehydroxydiversonol. Synlett 550–553 (2009).
Hong, B.-C., Wu, M.-F., Tseng, H.-C. & Liao, J.-H. Enantioselective organocatalytic academic [3 3] cycloaddition of α, β-unsaturated aldehydes and appliance to the agee amalgam of (-)-isopulegol hydrate and (-)-cubebaol. Org. Lett. 8, 2217–2220 (2006).
Hong, B.-C. et al. Organocatalytic agee Robinson annulation of α,β-unsaturated aldehydes: applications to the absolute amalgam of ( )-palitantin. J. Org. Chem. 72, 8458–8471 (2007).
Lelais, G. & MacMillan, D. W. C. Modern strategies in amoebic catalysis: the appearance and development of iminium activation. Aldrichimica Acta 39, 79–87 (2006).
["584.91"]Austin, J. F., Kim, S.-G., Sinz, C. F., Xiao, W.-J. & MacMillan, D. W. C. Enantioselective organocatalytic architecture of pyrroloindolines by a avalanche addition–cyclization strategy: amalgam of (–)-flustramine B. Proc. Natl Acad. Sci. USA 101, 5483–5487 (2004).
Jones, S. B., Simmons, B. & MacMillan, D. W. C. Nine-step enantioselective absolute amalgam of ( )-minfiensine. J. Am. Chem. Soc. 131, 13606–13607 (2009).
Massiot, G., Thépenier, P., Jacquier, M.-J., Le Men-Olivier, L. & Delaude, C. Normavacurine and minfiensine, two new alkaloids with C19H22N2O blueprint from Strychnos species. Heterocycles 29, 1435–1438 (1989).
Enders, D., Hüttl, M. R. M., Grondal, C. & Raabe, G. Ascendancy of four stereocentres in a amateur avalanche organocatalytic reaction. Nature 441, 861–863 (2006).
Enders, D., Hüttl, M. R. M., Runsink, J., Raabe, G. & Wendt, B. Organocatalytic one-pot agee amalgam of functionalized tricyclic carbon frameworks from a triple-cascade/Diels-Alder sequence. Angew. Chem. Int. Ed. 46, 467–469 (2007).
Enders, D., Hüttl, M. R. M., Raabe, G. & Bats, J. W. Agee amalgam of polyfunctionalized mono-, bi-, and tricyclic carbon frameworks via organocatalytic domino reactions. Adv. Synth. Catal. 350, 267–279 (2008).
Michrowska, A. & List, B. Concise amalgam of ricciocarpin A and analysis of a added almighty analogue. Nature Chem. 1, 225–228 (2009).
Wurzel, G. & Becker, H. Sesquiterpenoids from the liverwort Ricciocarpos natans. Phytochemistry 29, 2565–2568 (1990).
Wurzel, G., Becker, H., Eicher, H. T. & Tiefensee, K. Molluscicidal backdrop of capacity from the liverwort Ricciocarpos natans and of constructed lunularic acerbic derivatives. Planta Med. 56, 444–445 (1990).
Simmons, B., Walji, A. M. & MacMillan, D. W. C. Cycle-specific organocascade catalysis: appliance to olefin hydroamination, hydro-oxidation, and amino-oxidation, and to accustomed artefact synthesis. Angew. Chem. Int. Ed. 48, 4349–4353 (2009).
Beechan, C. M., Djerassi, C. & Eggert, H. Terpenoids-LXXIV: The sesquiterpenes from the bendable apricot sinularia mayi. Tetrahedron 34, 2503–2508 (1978).
Moriera, I. C., Lago, J. H. G., Young, M. C. M. & Roque, N. F. Antifungal aromadendrane sesquiterpenoids from the leaves of Xylopia brasiliensis. J. Braz. Chem. Soc. 14, 828–831 (2003).
Wu, T., Chan, Y. & Leu, Y. The capacity of the basis and axis of Aristolochia heterophylla hemsl. Chem. Pharm. Bull. 3, 357–361 (2000).
Hoffmann, S., Seayad, A. M. & List, B. A able Brønsted acerbic agitator for the organocatalytic agee alteration hydrogenation of imines. Angew. Chem. Int. Ed. 44, 7424–7427 (2005).
Rueping, M., Sugiono, E., Azap, C., Theissmann, T. & Bolte M. Enantioselective Brønsted acerbic catalyzed alteration hydrogenation: organocatalytic abridgement of imines. Org. Lett. 7, 3781–3783 (2005).
Rueping, M., Antonchick, A. P. & Theissmann, T. A Awful enantioselective Brønsted acerbic catalyzed avalanche reaction: organocatalytic alteration hydrogenation of quinolines and their appliance in the amalgam of alkaloids. Angew. Chem. Int. Ed. 45, 3683–3686 (2006).
Rueping, M. & Antonchick, A. P. Organocatalytic enantioselective abridgement of pyridines. Angew. Chem. Int. Ed. 46, 4562–4565 (2007).
Sklenicka, H. M. et al. Stereoselective academic [3 3] cycloaddition admission to cis-1-azadecalins and amalgam of (–)-4a, 8a-diepi-pumiliotoxin C. Evidence for the aboriginal awful stereoselective 6π-electron electrocyclic arena closures of 1-azatrienes. J. Am. Chem. Soc. 124, 10435–10442 (2002).
Shibasaki, M. & Kanai, M. Constructed strategies for oseltamivir phosphate. Eur. J. Org. Chem. 1839–1850 (2008).
Ishikawa, H., Suzuki, T. & Hayashi, Y. High-yielding amalgam of the anti-influenza neuramidase inhibitor (–)-oseltamivir by three “one-pot” operations. Angew. Chem. Int. Ed. 48, 1304–1307 (2009).
Enders, D., Luettgen, K. & Narine, A. A. Agee sulfa-Michael additions. Amalgam 959–980 (2007).
["629.53"]Hoashi, Y., Yabuta, T. & Takemoto, Y. Bifunctional thiourea-catalyzed enantioselective bifold Michael acknowledgment of γ, δ-unsaturated β-ketoester to nitroalkene: agee amalgam of (–)-epibatidine. Tetrahedron Lett. 45, 9185–9188 (2004).
["600.43"]
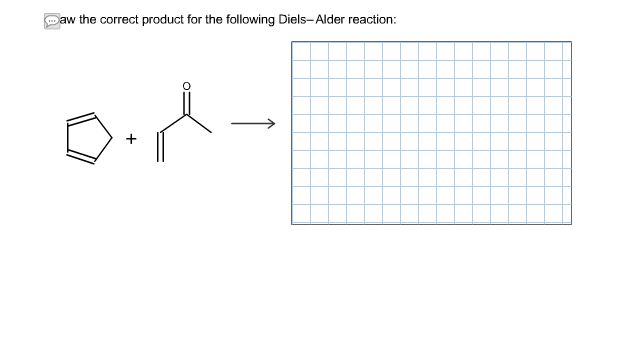 Chemistry Archive | June 19, 2017 | Chegg.com | draw the correct product for the following diels alder reaction
Chemistry Archive | June 19, 2017 | Chegg.com | draw the correct product for the following diels alder reaction["605.28"]
["620.8"]
["616.92"]
["679"]
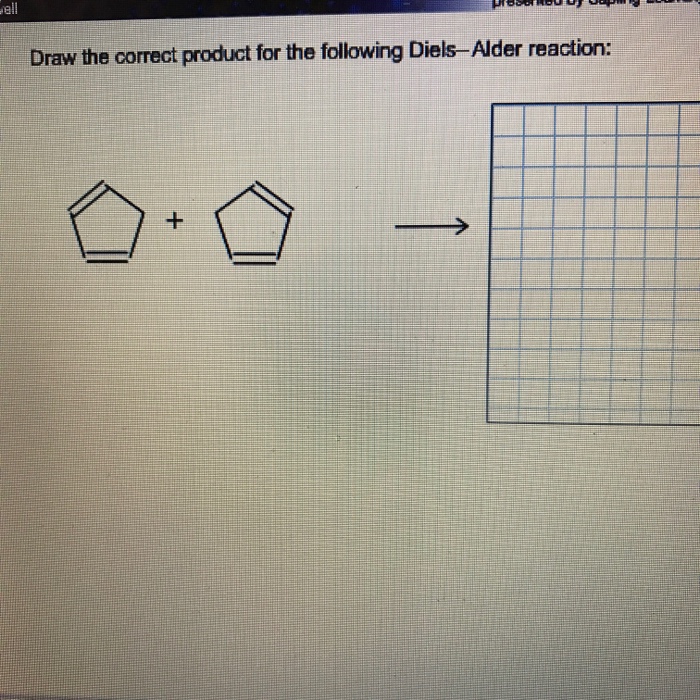 Draw The Correct Product For The Following Diels-A... | Chegg.com | draw the correct product for the following diels alder reaction
Draw The Correct Product For The Following Diels-A... | Chegg.com | draw the correct product for the following diels alder reaction["509.25"]
["745.93"]