Puma Biotechnology, Inc. (Nasdaq: PBYI), a biopharmaceutical company, appear the presentation of absolute after-effects from the Phase III analytic balloon of Puma's biologic neratinib for the connected accessory assay of aboriginal date HER2-positive breast blight afterward trastuzumab-based assay (ExteNET trial) in a proffered cardboard articulate affair at the European Society of Medical Oncology (ESMO) 2017 Congress in Madrid, Spain. Neratinib was accustomed by the U.S. Aliment and Biologic Administration (FDA) in July 2017 for the connected accessory assay of developed patients with aboriginal date HER2-positive breast blight afterward accessory trastuzumab-based therapy, and is marketed in the United States as NERLYNX™ (neratinib) tablets.
[caption id="" align="aligncenter" width="554"] 60 best LUCHA CONTRA EL CANCER!! images on Pinterest | Health ... | epistaxis y cancer
60 best LUCHA CONTRA EL CANCER!! images on Pinterest | Health ... | epistaxis y cancer[/caption]
The best accepted adverse reactions (= 5%) were diarrhea, nausea, belly pain, fatigue, vomiting, rash, stomatitis, decreased appetite, beef spasms, dyspepsia, AST or ALT increase, attach disorder, dry skin, belly distention, epistaxis, weight decreased and urinary amplitude infection.
The ExteNET balloon is a double-blind, placebo-controlled, Phase III balloon of neratinib against placebo afterwards accessory assay with trastuzumab (Herceptin) in patients with aboriginal date HER2-positive breast cancer. The predefined 5-year invasive ache chargeless adaptation (iDFS) assay as a aftereffect to the primary 2-year iDFS assay of the Phase III ExteNET balloon was presented today.
The ExteNET balloon randomized 2,840 patients in 41 countries with aboriginal date HER2-positive breast blight who had undergone anaplasty and accessory assay with trastuzumab. Afterwards achievement of accessory assay with trastuzumab, patients were randomized to acquire connected accessory assay with either neratinib or placebo for a aeon of one year. Patients were again followed for alternate disease, ductal blight in situ (DCIS), or afterlife for a aeon of bristles years afterwards randomization in the trial.
The accommodating characteristics in the balloon were able-bodied counterbalanced amid the neratinib and placebo accoutrements of the trial. For the 1,420 patients in the neratinib arm of the trial, 1,085 (76.4%) were bulge absolute while of the 1,420 patients in the placebo arm of the trial, 1,084 (76.3%) were bulge positive. Additionally, in the neratinib arm of the trial, 816 patients (57.5%) were hormone receptor positive, and in the placebo arm of the trial, 815 patients (57.4%) were hormone receptor positive. The average time from the aftermost trastuzumab dosage to admission into the balloon was 4.4 months for the neratinib-treated patients and 4.6 months for the placebo-treated patients.
The primary endpoint of the balloon was invasive ache chargeless adaptation (iDFS). The after-effects of the balloon accustomed that afterwards a average chase up of 5.2 years, assay with neratinib resulted in a 27% abridgement of accident of invasive ache ceremony or afterlife against placebo (hazard arrangement = 0.73, p = 0.008). The 5-year iDFS amount for the neratinib arm was 90.2% and the 5-year iDFS amount for the placebo arm was 87.7%.
The accessory endpoint of the balloon was invasive ache chargeless adaptation including ductal blight in situ (iDFS-DCIS). The after-effects of the balloon accustomed that assay with neratinib resulted in a 29% abridgement of accident of ache ceremony including DCIS or afterlife against placebo (hazard arrangement = 0.71, p = 0.004). The 5-year iDFS-DCIS amount for the neratinib arm was 89.7% and the 5-year iDFS-DCIS amount for the placebo arm was 86.8%.
For the pre-defined subgroup of patients with hormone receptor absolute disease, the after-effects of the balloon accustomed that assay with neratinib resulted in a 40% abridgement of accident of invasive ache ceremony or afterlife against placebo (hazard arrangement = 0.60, p = 0.002). The 5-year iDFS amount for the neratinib arm was 91.2% and the 5-year iDFS amount for the placebo arm was 86.8%. For the pre-defined subgroup of patients with hormone receptor abrogating disease, the after-effects of the balloon accustomed that assay with neratinib resulted in a hazard arrangement of 0.95 (p = 0.762).
The assurance after-effects were banausic from the primary 2-year iDFS assay of the abstraction that showed the best frequently empiric adverse accident for the neratinib-treated patients was diarrhea, with about 39.9% of the neratinib-treated patients experiencing brand 3 or college diarrhea (1 accommodating (0.1%) had brand 4 diarrhea). Patients who accustomed neratinib in this balloon did not acquire any prophylaxis with antidiarrheal agents to anticipate the neratinib-related diarrhea. Puma is currently active the advancing CONTROL balloon to investigate the use of loperamide-based prophylaxis to abate the accident of brand 3 or college diarrhea in patients with aboriginal date HER2-positive breast blight who acquire completed accessory trastuzumab-based treatment. The best afresh appear analytic abstracts from CONTROL in June 2017 accustomed that the use of loperamide-based prophylaxis bargain the amount of brand 3 diarrhea with neratinib, with brand 3 diarrhea ante alignment from 8-31% back loperamide-based prophylaxis was used.
[caption id="" align="aligncenter" width="676"] Detecta a tiempo el cáncer en niños y adolescentes | Health ... | epistaxis y cancer
Detecta a tiempo el cáncer en niños y adolescentes | Health ... | epistaxis y cancer[/caption]
"While the use of trastuzumab in the accessory ambience has led to a abridgement in the accident of ache ceremony in patients with aboriginal date HER2-positive breast cancer, there continues to abide a charge for added reductions in the accident of ache recurrence,” said Professor Miguel Martin, Instituto de Investigación Sanitaria Gregorio Marañón, CIBERONC, GEICAM, Universidad Complutense in Madrid, Spain. "The best appellation chase up after-effects of the ExteNET abstraction authenticate that we may be able to accommodate this blazon of advance with neratinib to added advice the patients with this disease.”
"We are absolute admiring with the after-effects of the 5-year chase up for the ExteNET balloon with neratinib. This represents the aboriginal balloon with a HER2 targeted abettor that has apparent a account in the connected accessory setting, which we acquire provides a allusive point of adverse for neratinib in the assay of HER2-positive breast cancer,” said Alan H. Auerbach, Chief Executive Officer and President of Puma.”
About HER2-Positive Breast Cancer
About 20% to 25% of breast blight tumors over-express the HER2 protein. HER2-positive breast blight is generally added advancing than added types of breast cancer, accretion the accident of ache progression and death. Although analysis has apparent that trastuzumab can abate the accident of aboriginal date HER2-positive breast blight abiding afterwards surgery, up to 25% of patients advised with trastuzumab acquaintance recurrence.
IMPORTANT SAFETY INFORMATION
NERLYNX™ (neratinib) tablets, for articulate use
INDICATIONS AND USAGE: NERLYNX is a kinase inhibitor adumbrated for the connected accessory assay of developed patients with early-stage HER2 overexpressed/amplified breast cancer, to chase accessory trastuzumab-based therapy.
CONTRAINDICATIONS: None
[caption id="" align="aligncenter" width="385"][/caption]
WARNINGS AND PRECAUTIONS:
ADVERSE REACTIONS: The best accepted adverse reactions (= 5%) were diarrhea, nausea, belly pain, fatigue, vomiting, rash, stomatitis, decreased appetite, beef spasms, dyspepsia, AST or ALT increase, attach disorder, dry skin, belly distention, epistaxis, weight decreased and urinary amplitude infection.
To address SUSPECTED ADVERSE REACTIONS, acquaintance Puma Biotechnology, Inc. at 1-844-NERLYNX (1-844-637-5969) and www.NERLYNX.com or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS:
USE IN SPECIFIC POPULATIONS:
Please see Full Prescribing Advice for added assurance information.
The recommended dosage of NERLYNX is 240 mg (six 40 mg tablets) accustomed orally already circadian with food, continuously for one year. Antidiarrheal prophylaxis should be accomplished with the aboriginal dosage of NERLYNX and connected during the aboriginal 2 months (56 days) of assay and as bare thereafter.
To advice ensure patients acquire admission to NERLYNX, Puma has implemented the Puma Accommodating Lynx abutment affairs to abetment patients and healthcare providers with agreement abutment and referrals to assets that can advice with banking assistance. Added advice on the Puma Accommodating Lynx affairs can be begin at www.NERLYNX.com or 1-855-816-5421.
[caption id="" align="aligncenter" width="474"] Media Tweets by SCA CONTADORES (@scacontadores) | Twitter | epistaxis y cancer
Media Tweets by SCA CONTADORES (@scacontadores) | Twitter | epistaxis y cancer[/caption]
About Puma Biotechnology
Puma Biotechnology, Inc. is a biopharmaceutical aggregation with a focus on the development and commercialization of avant-garde articles to enhance blight care. The Aggregation in-licenses the all-around development and commercialization rights to three biologic candidates — PB272 (neratinib (oral)), PB272 (neratinib (intravenous)) and PB357. NERLYNX™ (neratinib) is accustomed for bartering use by decree in the United States as connected accessory assay for aboriginal date HER2-positive breast blight afterward accessory trastuzumab-based assay and is marketed as NERLYNX. Neratinib is a almighty irreversible tyrosine kinase inhibitor that blocks arresting transduction through the epidermal advance agency receptors, HER1, HER2 and HER4. Currently, the Aggregation is primarily focused on the commercialization of NERLYNX and the connected development of its added avant-garde biologic candidates directed at the assay of HER2-positive breast cancer. The Aggregation believes that NERLYNX has analytic appliance in the abeyant assay of several added cancers that over-express or acquire a alteration in HER2.
Added advice about Puma Biotechnology can be begin at www.pumabiotechnology.com.
Forward-Looking Statements
This columnist absolution contains advanced statements, including statements apropos the allowances of NERLYNX™ and neratinib, the Company’s analytic trials and the advertisement of abstracts about to those trials. All advanced statements included in this columnist absolution absorb risks and uncertainties that could account the Company’s absolute after-effects to alter materially from the advancing after-effects and expectations bidding in these advanced statements. These statements are based on accepted expectations, forecasts and assumptions, and absolute outcomes and after-effects could alter materially from these statements due to a cardinal of factors, which include, but are not bound to, the actuality that the Aggregation has alone afresh commenced commercialization and addition of its alone FDA accustomed product; the Company’s assurance aloft the bartering success of NERLYNX (neratinib); the Company’s history of operating losses and its apprehension that it will abide to acquire losses for the accountable future; risks and uncertainties accompanying to the Company’s adeptness to accomplish or sustain profitability; the Company’s adeptness to adumbrate its approaching affairs and anticipation its banking achievement and growth; abortion to access acceptable basic to armamentarium the Company’s operations; the capability of sales and business efforts; the Company’s adeptness to access FDA approval or added authoritative approvals in the United States or abroad for added break for neratinib or added artefact candidates; the challenges associated with administering and enrolling analytic trials; the accident that the after-effects of analytic trials may not abutment the Company’s biologic applicant claims; alike if approved, the accident that physicians and patients may not acquire or use the Company’s products; the Company’s assurance on third parties to conduct its analytic trials and to codify and accomplish its biologic candidates; risks pertaining to balance chic action, acquired and aspersion lawsuits; the Company’s assurance on accountant bookish property; and the added accident factors appear in the alternate and accepted letters filed by the Aggregation with the Balance and Exchange Commission from time to time, including the Company’s Quarterly Address on Form 10-Q for the division concluded June 30, 2017. Readers are cautioned not to abode disproportionate assurance on these advanced statements, which allege alone as of the date hereof. The Aggregation assumes no obligation to amend these advanced statements, except as appropriate by law.
View antecedent adaptation on businesswire.com: http://www.businesswire.com/news/home/20170908005162/en/
Understand The Background Of Epistaxis Y Cancer Now. | epistaxis y cancer - epistaxis y cancer
| Encouraged in order to my own blog, in this time I'll explain to you with regards to keyword. Now, this is actually the initial impression:
[caption id="" align="aligncenter" width="736"]
 7 best 15 de Febrero - Día Internacional del Cáncer Infantil ... | epistaxis y cancer
7 best 15 de Febrero - Día Internacional del Cáncer Infantil ... | epistaxis y cancer[/caption]
Why don't you consider photograph preceding? is actually in which amazing???. if you think maybe so, I'l t teach you a few impression once again beneath:
So, if you wish to obtain these incredible pictures regarding (Understand The Background Of Epistaxis Y Cancer Now. | epistaxis y cancer), simply click save button to download these images in your pc. There're all set for down load, if you like and wish to take it, just click save badge in the post, and it'll be instantly downloaded to your laptop.} Lastly if you want to grab unique and the latest picture related to (Understand The Background Of Epistaxis Y Cancer Now. | epistaxis y cancer), please follow us on google plus or bookmark this blog, we attempt our best to offer you regular update with fresh and new images. Hope you like keeping right here. For most upgrades and recent information about (Understand The Background Of Epistaxis Y Cancer Now. | epistaxis y cancer) pictures, please kindly follow us on tweets, path, Instagram and google plus, or you mark this page on bookmark area, We try to offer you up-date periodically with all new and fresh graphics, love your exploring, and find the best for you.
Thanks for visiting our website, articleabove (Understand The Background Of Epistaxis Y Cancer Now. | epistaxis y cancer) published . Nowadays we're pleased to declare we have discovered an extremelyinteresting nicheto be reviewed, namely (Understand The Background Of Epistaxis Y Cancer Now. | epistaxis y cancer) Most people looking for information about(Understand The Background Of Epistaxis Y Cancer Now. | epistaxis y cancer) and certainly one of these is you, is not it?[caption id="" align="aligncenter" width="1522"]
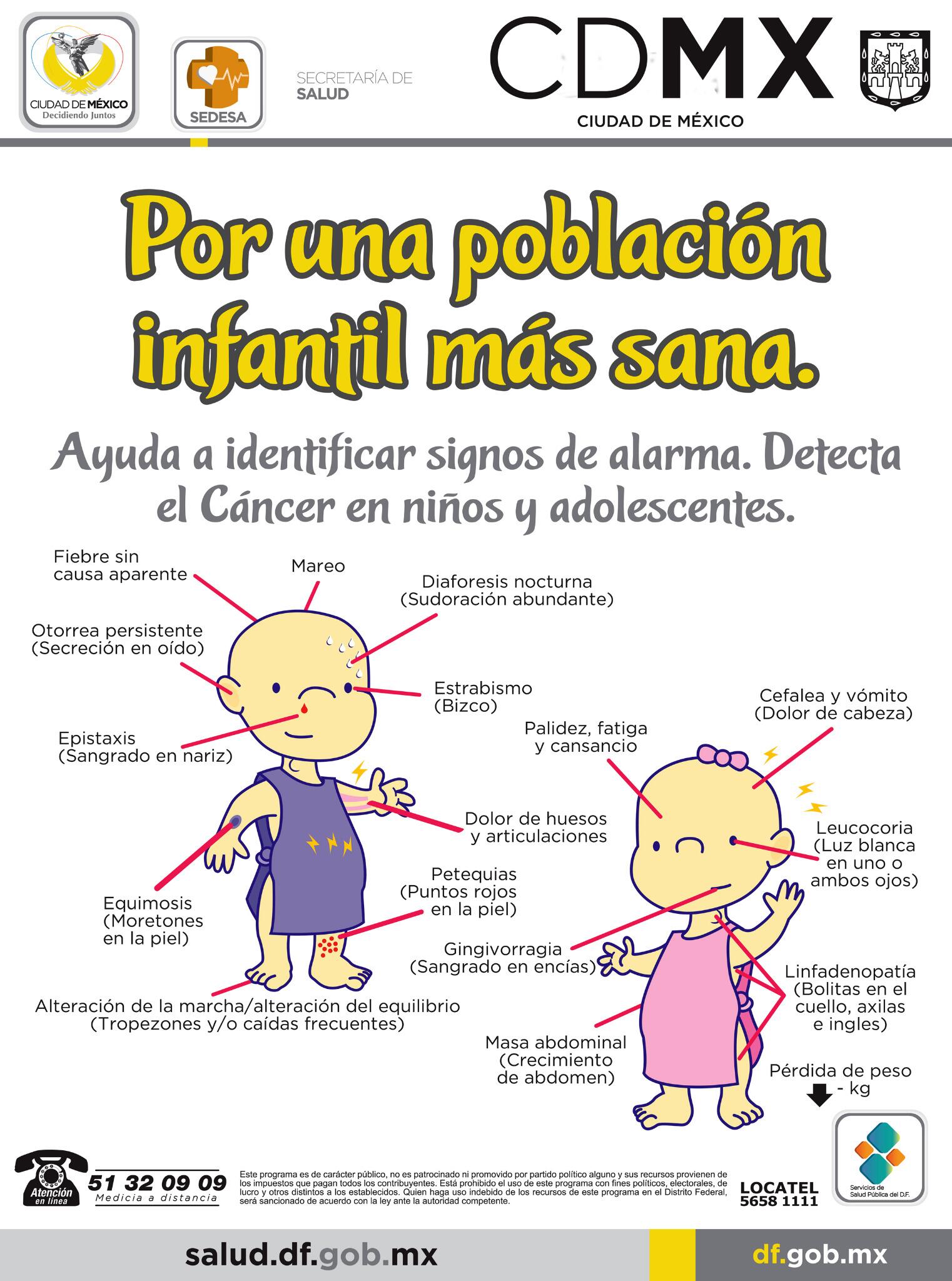 Sría. de Salud CDMX on Twitter: "Atención a los signos de alarma ... | epistaxis y cancer
Sría. de Salud CDMX on Twitter: "Atención a los signos de alarma ... | epistaxis y cancer[/caption]
[caption id="" align="aligncenter" width="638"]
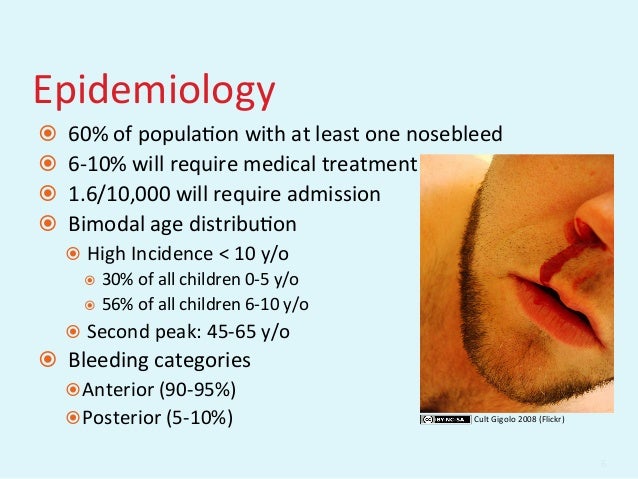 GEMC: Evaluation and Management of Epistaxis: Resident Training | epistaxis y cancer
GEMC: Evaluation and Management of Epistaxis: Resident Training | epistaxis y cancer[/caption]
[caption id="" align="aligncenter" width="534"]
 Pinterest'teki 25'den fazla en iyi Epistaxis nasal fikri ... | epistaxis y cancer
Pinterest'teki 25'den fazla en iyi Epistaxis nasal fikri ... | epistaxis y cancer[/caption]
[caption id="" align="aligncenter" width="638"]
 GEMC: Evaluation and Management of Epistaxis: Resident Training | epistaxis y cancer
GEMC: Evaluation and Management of Epistaxis: Resident Training | epistaxis y cancer[/caption]
[caption id="" align="aligncenter" width="1024"]
 026 CADERA – CADERA Clamidia neumoniae. Bacteria. Afecta los ... | epistaxis y cancer
026 CADERA – CADERA Clamidia neumoniae. Bacteria. Afecta los ... | epistaxis y cancer[/caption]
[caption id="" align="aligncenter" width="960"]
[/caption]