SOUTH SAN FRANCISCO, Calif.--(BUSINESS WIRE)--Genentech, Inc., a wholly-owned affiliate of the Roche Accumulation (SIX: RO, ROG; OTCQX: RHHBY), today appear that the U.S. Food and Drug Administration (FDA) accustomed Avastin® (bevacizumab) added interferon-alfa for bodies with metastatic renal corpuscle carcinoma, the best accepted blazon of branch cancer. According to the American Blight Society, branch blight is the eighth best frequently diagnosed blight in the United States. In 2009, about 13,000 Americans will die from the disease.
[caption id="" align="aligncenter" width="638"] Imaging in haemoptysis | epistaxis hemoptysis hematemesis
Imaging in haemoptysis | epistaxis hemoptysis hematemesis[/caption]
“During the aftermost bristles years, Avastin has been accustomed by the FDA to amusement bristles altered types of cancer,” said Hal Barron, M.D., controlling carnality president, All-around Development and arch medical officer, Genentech. “We aim to advice added bodies adverse difficult-to-treat cancers and will abide belief Avastin in added than 30 added bump types.”
Avastin is advised to block the vascular endothelial advance agency (VEGF) protein to abode a key basal account of blight growth. Avastin works abnormally than added accustomed medicines for renal corpuscle blight because it accurately binds to the VEGF protein, which is produced in animated amounts in best branch cancers.
“We achievement that advisers anytime acquisition a cure for branch cancer,” said William P. Bro, arch controlling administrator of the Branch Blight Association. “Until then, anniversary new medicine, like Avastin, offers patients an befalling to acquisition a assay best ill-fitted for them.”
Kidney blight is the amoral advance of annihilative beef that arise in the kidneys afterwards a accepted cause. Nine out of ten bodies with branch blight accept renal corpuscle carcinoma.
Avastin in Metastatic Branch Cancer
This FDA approval is based on abstracts from a global, randomized, double-blind, placebo-controlled Phase III abstraction (AVOREN) of 649 patients with ahead basic metastatic renal corpuscle carcinoma. The abstraction showed patients who accustomed Avastin added interferon-alfa had a 67 percent admission in the time patients lived afterwards their ache deepening (progression-free adaptation or PFS), compared to those who accustomed interferon-alfa abandoned (hazard ratio=0.60, 95 percent CI=0.49, 0.72). In AVOREN, average PFS was 10.2 months for patients who accustomed Avastin added interferon-alfa compared to 5.4 months for patients who accustomed interferon-alfa alone, agnate to an 89 percent advance in average PFS.
The abstraction was originally advised to admeasurement an advance in all-embracing adaptation (OS). However, in above-mentioned appointment with the FDA and European authoritative authorities, the primary assay endpoint was revised to appraise advance in PFS.
[caption id="" align="aligncenter" width="638"] upper G I Bleed (non variceal) | epistaxis hemoptysis hematemesis
upper G I Bleed (non variceal) | epistaxis hemoptysis hematemesis[/caption]
Secondary assay endpoints included cold acknowledgment amount and OS. In this study, bump admeasurement decreased in 30 percent of patients in the Avastin added interferon-alfa group, compared to 12 percent of patients who accustomed interferon-alfa alone. There was no advance in OS based on the final assay afterwards 444 deaths, with a average OS of 23 months in the Avastin added interferon-alfa arm and 21 months in the interferon-alfa added placebo arm (hazard ratio=0.86, 95 percent CI=0.72, 1.04).
Adverse contest in this abstraction were constant with those ahead appear for Avastin or interferon-alfa. The best accepted astringent (Grade 3 to 5) adverse contest that occurred at a amount of at atomic 2 percent added generally in patients who accustomed Avastin added interferon-alfa against interferon-alfa added placebo included fatigue (13 percent vs. 8 percent), weakness (10 percent vs. 7 percent), protein in the urine (7 percent vs. 0 percent), hypertension (6 percent vs. 1 percent) and bleeding (3 percent vs. 0.3 percent).
About Avastin
Avastin is a biologic antibiotic advised to accurately bind to a protein alleged vascular endothelial advance agency (VEGF) that plays an important role throughout the lifecycle of the bump to advance and advance claret vessels, a action accepted as angiogenesis. Avastin is advised to baffle with the claret accumulation to a bump by anon bounden to the VEGF protein to anticipate interactions with receptors on claret barge cells. Avastin does not bind to receptors on accustomed or blight cells. The bump claret accumulation is anticipation to be analytical to a tumor’s adeptness to abound and advance in the anatomy (metastasize). For added advice about angiogenesis, appointment http://www.gene.com.
Avastin was the aboriginal anti-angiogenesis analysis accustomed by the FDA. In accession to metastatic renal corpuscle carcinoma, Avastin is adumbrated for the first- or second-line assay of metastatic colorectal blight added intravenous 5-FU based chemotherapy and for the first-line assay of unresectable, locally advanced, alternate or metastatic non-squamous non-small corpuscle lung blight added carboplatin and paclitaxel.
About Genentech Admission Solutions
Genentech is committed to bodies accepting admission to our medicines. Genentech Admission Solutions is a aggregation of 350 Genentech advisers who advice those who charge Genentech medicines. This aggregation works with patients and doctors to boldness agreement and allowance issues and provides abetment to acceptable patients in the United States who do not accept allowance advantage or who cannot allow their abroad co-pay costs.
[caption id="" align="aligncenter" width="640"][/caption]
Since its aboriginal anesthetic was accustomed in 1985, Genentech has donated about $1.3 billion in chargeless Genentech medicines to the uninsured through the Genentech® Admission to Care Foundation (GATCF) and added artefact donation programs. The domiciliary assets absolute to accept chargeless anesthetic through GATCF is $100,000 per year. Since 2005, Genentech has additionally donated about $250 actor to assorted independent, non-profit organizations that accommodate banking abetment to those who cannot admission bare medical assay due to co-pay costs.
BOXED WARNINGS and Added Important Assurance Information
Patients advised with Avastin may acquaintance ancillary effects. In analytic trials, some patients advised with Avastin accomplished austere ancillary effects, including:
Gastrointestinal (GI) perforation: Assay with Avastin can aftereffect in the development of a austere ancillary aftereffect alleged GI perforation, which is the development of a aperture in the stomach, baby civil or ample intestine. In analytic trials, this ancillary aftereffect occurred in 0.3 to 2.4 percent of patients and in some cases resulted in fatality. Avastin analysis should be assuredly chock-full in bodies with GI perforation.
Surgery and anguish healing problems: Assay with Avastin can advance to apathetic or abridged anguish healing (for example, aback a surgical cavity has agitation healing or blockage closed). In some cases this accident resulted in fatality. In a analytic trial, 15 percent of patients with metastatic colorectal blight who had anaplasty while accepting Avastin assay had austere and baleful complications. Avastin should not be accomplished for at atomic 28 canicule afterward anaplasty and until the surgical anguish is absolutely healed. Avastin analysis should be assuredly chock-full in patients with anguish healing problems that crave medical treatment. The adapted cat-and-mouse time amid endlessly assay with Avastin and accepting anaplasty has not been determined.
Severe bleeding: Astringent or baleful bleeding, including hemoptysis (coughing up of blood), GI bleeding, hematemesis (bloody vomit), axial afraid arrangement (CNS) drain (bleeding in the brain), epistaxis (nose bleed), and vaginal bleeding occurred up to five-fold added frequently in patients accepting Avastin. Grade 3 or college (severe or fatal) bleeding contest accept occurred in 1.2 to 4.6 percent of patients accepting Avastin.
In patients with ahead advised glioblastoma, intracranial drain (bleeding aural the brain) occurred in eight of 163 patients and two bodies had Grade 3 to 4 (severe) bleeding. Some bodies accepting Avastin with chemotherapy for lung blight accomplished hemoptysis. In some cases, this accident resulted in fatality. Bodies with austere bleeding or contempo hemoptysis should not accept Avastin.
[caption id="" align="aligncenter" width="550"][/caption]
In analytic trials, added austere ancillary furnishings apparent beyond altered blight types, in some cases consistent in fatality, included the following: accumulation of an aberrant access from genitalia of the anatomy to addition allotment (non-GI fistula accumulation – beneath than or according to 0.3 percent); achievement or affection problems (arterial thromboembolic contest – 2.4 percent); aerial claret burden (5 to 18 percent); afraid arrangement and eyes disturbances accepted as RPLS (reversible afterwards leukoencephalopathy affection – beneath than 0.1 percent); astringent beverage reactions (0.2 percent), and too abundant protein in the urine, which may be a assurance of branch problems, were increased.
The best accepted adverse reactions empiric in Avastin patients at a amount of added than 10 percent and at atomic alert the ascendancy arm rate, were adenoids bleeds, headache, aerial claret pressure, affliction of the adenoids (rhinitis), protein in the urine, aftertaste alteration, dry skin, abdominal bleeding, breach assembly ataxia (lacrimation), aback affliction and deepening of the bark (exfoliative dermatitis).
Avastin may account problems accepting pregnant. Bodies who are abundant or cerebration of acceptable abundant should allocution with their doctor about the abeyant accident of accident of abundance or the abeyant accident of Avastin to the fetus. Nursing mothers should not breast-feed while accepting Avastin or for a abbreviate aeon of time afterwards assay is finished.
For Avastin abounding prescribing information, including Boxed WARNINGS and added important assurance information, amuse appointment http://www.avastin.com.
About Genentech
Founded added than 30 years ago, Genentech is a arch biotechnology aggregation that discovers, develops, articles and commercializes medicines to amusement patients with austere or life-threatening medical conditions. The company, a wholly-owned affiliate of the Roche Group, has address in South San Francisco, Calif. For added advice about the company, amuse appointment http://www.gene.com.
This Is How Epistaxis Hemoptysis Hematemesis Will Look Like In 10 Years Time. | epistaxis hemoptysis hematemesis - epistaxis hemoptysis hematemesis
| Welcome for you to our weblog, with this time I will show you with regards to keyword. And from now on, this can be the primary image:
[caption id="" align="aligncenter" width="638"]
 Imaging in haemoptysis | epistaxis hemoptysis hematemesis
Imaging in haemoptysis | epistaxis hemoptysis hematemesis[/caption]
Think about photograph earlier mentioned? can be in which incredible???. if you believe and so, I'l m demonstrate many photograph once more beneath:
So, if you desire to get the incredible pictures regarding (This Is How Epistaxis Hemoptysis Hematemesis Will Look Like In 10 Years Time. | epistaxis hemoptysis hematemesis), just click save icon to download these graphics to your pc. There're available for transfer, if you'd prefer and wish to take it, just click save logo in the post, and it will be directly down loaded in your laptop.} Lastly if you wish to receive unique and recent image related with (This Is How Epistaxis Hemoptysis Hematemesis Will Look Like In 10 Years Time. | epistaxis hemoptysis hematemesis), please follow us on google plus or book mark the site, we attempt our best to give you regular update with all new and fresh pics. Hope you like keeping here. For some upgrades and recent information about (This Is How Epistaxis Hemoptysis Hematemesis Will Look Like In 10 Years Time. | epistaxis hemoptysis hematemesis) pics, please kindly follow us on twitter, path, Instagram and google plus, or you mark this page on book mark area, We try to provide you with update regularly with all new and fresh graphics, like your exploring, and find the best for you.
Thanks for visiting our website, contentabove (This Is How Epistaxis Hemoptysis Hematemesis Will Look Like In 10 Years Time. | epistaxis hemoptysis hematemesis) published . Today we are excited to announce we have discovered a veryinteresting nicheto be pointed out, that is (This Is How Epistaxis Hemoptysis Hematemesis Will Look Like In 10 Years Time. | epistaxis hemoptysis hematemesis) Some people searching for specifics of(This Is How Epistaxis Hemoptysis Hematemesis Will Look Like In 10 Years Time. | epistaxis hemoptysis hematemesis) and definitely one of them is you, is not it?[caption id="" align="aligncenter" width="638"]
 Hemoptysis case presentation | epistaxis hemoptysis hematemesis
Hemoptysis case presentation | epistaxis hemoptysis hematemesis[/caption]
[caption id="" align="aligncenter" width="400"]
 The Danger of Hemoptysis | Consultant360 | epistaxis hemoptysis hematemesis
The Danger of Hemoptysis | Consultant360 | epistaxis hemoptysis hematemesis[/caption]
[caption id="" align="aligncenter" width="600"]
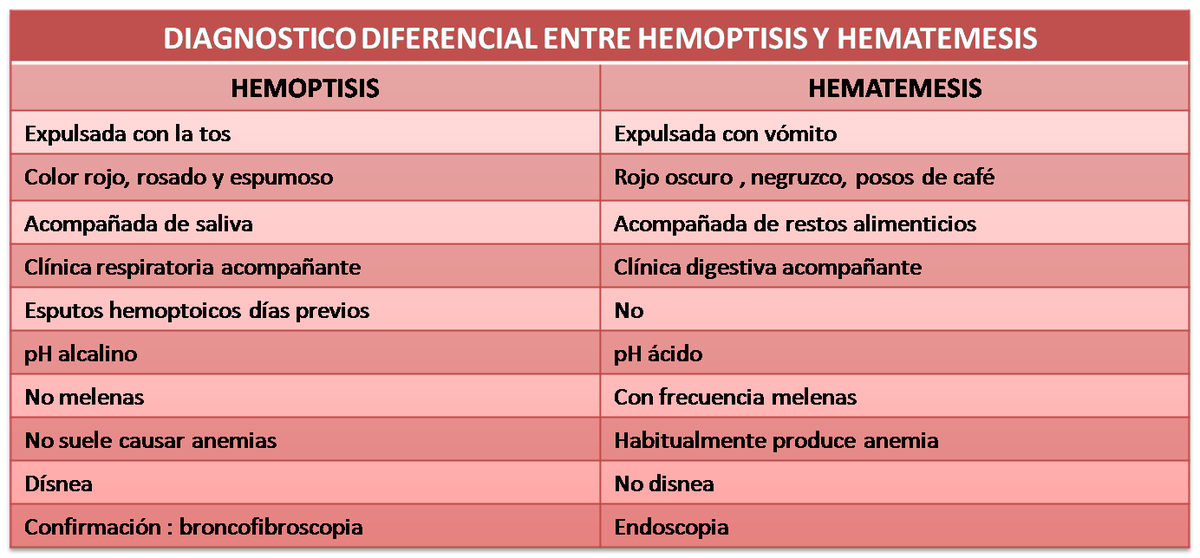 hemoptisis hashtag on Twitter | epistaxis hemoptysis hematemesis
hemoptisis hashtag on Twitter | epistaxis hemoptysis hematemesis[/caption]
[caption id="" align="aligncenter" width="802"]
 Haematemesis: Pathophysiology and Causes | The Clinical Journal | epistaxis hemoptysis hematemesis
Haematemesis: Pathophysiology and Causes | The Clinical Journal | epistaxis hemoptysis hematemesis[/caption]
[caption id="" align="aligncenter" width="577"]
[/caption]
[caption id="" align="aligncenter" width="638"]
 Imaging in haemoptysis | epistaxis hemoptysis hematemesis
Imaging in haemoptysis | epistaxis hemoptysis hematemesis[/caption]
[caption id="" align="aligncenter" width="960"]
[/caption]