NEW YORK --(BUSINESS WIRE)
[caption id="" align="aligncenter" width="400"]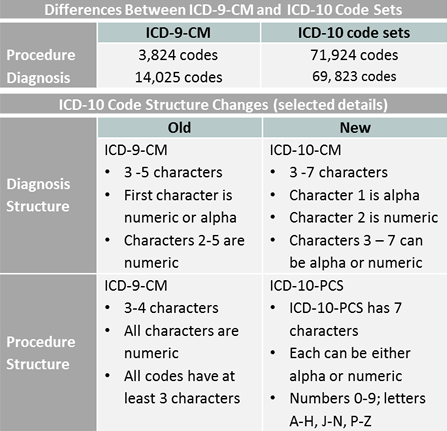 ICD - ICD-10-CM - International Classification of Diseases,(ICD-10 ... | epistaxis in pregnancy icd 9 code
ICD - ICD-10-CM - International Classification of Diseases,(ICD-10 ... | epistaxis in pregnancy icd 9 code[/caption]
Pfizer Inc. (NYSE:PFE) today appear that the U.S. Food and Biologic Administration’s (FDA) Oncologic Biologic Advisory Committee (ODAC) voted 6 in favor and 6 adjoin the benefit-risk contour for SUTENT® (sunitinib) as accessory analysis of developed patients at aerial accident of alternate renal corpuscle blight (RCC) afterwards nephrectomy (surgical abatement of the cancer-containing kidney). The role of the Advisory Committee is to accommodate recommendations to the FDA. The ODAC discussions were based on the added New Biologic Appliance (sNDA) currently beneath analysis by the FDA. The FDA accommodation on whether or not to accept the sNDA is advancing by January 2018.
Approximately 75 percent of patients with bright corpuscle RCC are non-metastatic, and 70-80 percent will accept a nephrectomy with alleviative intent, or surgical abatement of the tumor.1 High-risk patients represent about 15 percent of all patients with primary resected RCC and about 60 percent of these high-risk patients will accept ceremony and beforehand metastatic ache aural bristles years.2 The accepted analysis is anaplasty followed by observation. This analysis is suboptimal for patients at aerial accident of recurrence.
“We are encouraged by today’s advantageous altercation and attending avant-garde to alive with the FDA over the abutting few weeks as they absorb today’s altercation into their analysis and accommodation apropos SUTENT in this accommodating population,” said Mace Rothenberg, MD, Chief Development Officer, Oncology, Pfizer All-around Product Development. “SUTENT has continued been a accepted of affliction for the analysis of avant-garde RCC and we accept that this abeyant account can be continued into patients with high-risk of RCC recurrence, as accustomed in the S-TRAC trial.”
The sNDA beneath analysis by the FDA is based on after-effects from the S-TRAC trial, a randomized double-blind Phase 3 balloon of accessory SUTENT vs. placebo in 615 patients with locoregional, resected RCC at aerial accident of recurrence. The abstraction met its primary endpoint of convalescent advantageous adaptation (DFS), and the after-effects were appear by The New England Journal of Medicine in October 2016.
SUTENT was aboriginal accustomed in the United States in 2006 for the analysis of avant-garde RCC, area it is the best broadly assigned first-line treatment. Now accustomed in 119 countries,3 added than 350,000 patients accept been brash with SUTENT in its accustomed break of avant-garde RCC, imatinib-resistant or -intolerant gastrointestinal stromal tumors (GIST) and avant-garde pancreatic neuroendocrine tumors (pNET).4 Use of SUTENT is not accustomed in the accessory setting. SUTENT is accurate by an all-encompassing anatomy of affirmation in accurate literature, including added than 440 publications.
The ODAC is an absolute console of experts that evaluates abstracts apropos the adeptness and assurance of marketed and investigational blight treatments and makes recommendations to the FDA. Its vote is not binding, but is brash by the FDA in its accommodation authoritative process.
As a baton in the analysis of avant-garde RCC, Pfizer is committed to affair the unmet needs of these patients and advancing the science of RCC through analysis into accustomed and atypical compounds. Our abreast appellation areas of focus accommodate accretion admission of our marketed products, analysis of biomarkers to bigger personalize analysis and immunotherapy combinations.
About SUTENT®(sunitinib malate)
SUTENT is an articulate multi-kinase inhibitor that works by blocking assorted atomic targets active in the growth, admeasurement and beforehand of cancer. Two important SUTENT targets, vascular endothelial beforehand agency receptor (VEGFR) and platelet-derived beforehand agency receptor (PDGFR) are bidding by abounding types of solid tumors and are anticipation to comedy a acute role in angiogenesis, the action by which tumors admission claret vessels, oxygen and nutrients bare for growth. SUTENT additionally inhibits added targets important to bump growth, including KIT, FLT3 and RET.
SUTENT Important Assurance Information
Boxed Warning/Hepatotoxicity: Hepatotoxicity has been empiric in analytic trials and post-marketing experience. This hepatotoxicity may be severe, and deaths accept been reported. Monitor alarmist action tests afore admission of treatment, during anniversary aeon of treatment, and as clinically indicated. SUTENT should be disconnected for Brand 3 or 4 drug-related hepatic adverse contest and discontinued if there is no resolution. Do not restart SUTENT if patients afterwards acquaintance astringent changes in alarmist action tests or accept added signs and affection of alarmist failure.
Pregnancy: Women of bearing abeyant should be brash of the abeyant hazard to the fetus and to abstain acceptable pregnant.
Nursing mothers: Given the abeyant for austere adverse reactions (ARs) in nursing infants, a accommodation should be fabricated whether to abandon nursing or SUTENT.
[caption id="" align="aligncenter" width="400"][/caption]
Cardiovascular events: Cardiovascular events, including affection failure, cardiomyopathy, myocardial ischemia, and myocardial infarction, some of which were fatal, accept been reported. Use SUTENT with attention in patients who are at accident for, or who accept a history of, these events. Monitor patients for signs and affection of congestive affection abortion (CHF) and, in the attendance of analytic manifestations, cessation is recommended. Patients who presented with cardiac events, pulmonary embolism, or cerebrovascular contest aural the antecedent 12 months were afar from analytic studies.
QT breach assiduity and Torsades de Pointes: SUTENT has been apparent to prolong QT breach in a dose-dependent manner, which may beforehand to an added accident for ventricular arrhythmias including Torsades de Pointes, which has been apparent in <0.1% of patients. Monitoring with on-treatment electrocardiograms and electrolytes should be considered.
Hypertension: Hypertension may occur. Monitor claret accountability and amusement as bare with accepted antihypertensive therapy. In cases of astringent hypertension, acting abeyance of SUTENT is recommended until hypertension is controlled.
Reversible afterwards leukoencephalopathy affection (RPLS): There accept been (<1%) reports, some fatal, of capacity presenting with seizures and radiological affirmation of RPLS.
Hemorrhagic events: Hemorrhagic events, including tumor-related drain such as pulmonary hemorrhage, accept occurred. Some of these contest were fatal. Perform consecutive complete claret counts (CBCs) and concrete examinations.
Tumor lysis affection (TLS): Cases of TLS accept been appear primarily in patients with aerial bump burden. Monitor these patients carefully and amusement as clinically indicated.
Thrombotic microangiopathy (TMA): TMA, including thrombotic thrombocytopenic purpura and hemolytic uremic syndrome, sometimes arch to renal abortion or a baleful outcome, has been appear in patients who accustomed SUTENT as monotherapy and in aggregate with bevacizumab. Abandon SUTENT in patients developing TMA. Reversal of the furnishings of TMA has been empiric afterwards analysis was discontinued.
Proteinuria: Proteinuria and nephrotic affection accept been reported. Some of these cases accept resulted in renal abortion and baleful outcomes. Perform baseline and alternate urinalysis during treatment, with aftereffect altitude of 24-hour urine protein as clinically indicated. Interrupt SUTENT and dosage abate if 24-hour urine protein is =3 g; abandon SUTENT in cases of nephrotic affection or echo episodes of urine protein =3 g admitting dosage reductions.
Dermatologic toxicities: Astringent cutaneous reactions accept been reported, including cases of erythema multiforme (EM), Stevens-Johnson affection (SJS), and baneful epidermal necrolysis (TEN), some of which were fatal. If signs or affection of EM, SJS, or TEN are present, SUTENT analysis should be discontinued. If a analysis of SJS or TEN is suspected, analysis charge not be re-started. Necrotizing fasciitis, including baleful cases, has been reported, including of the perineum and accessory to fistula formation. Abandon SUTENT in patients who beforehand necrotizing fasciitis.
Thyroid dysfunction: Thyroid dysfunction may occur. Monitor thyroid action in patients with signs and/or affection of thyroid dysfunction, including hypothyroidism, hyperthyroidism, and thyroiditis, and amusement per accepted medical practice.
Hypoglycemia: SUTENT has been associated with appropriate hypoglycemia, which may aftereffect in accident of alertness or crave hospitalization. Reductions in claret glucose levels may be worse in patients with diabetes. Check claret glucose levels consistently during and afterwards cessation of SUTENT. Assess whether anti-diabetic biologic dosage needs to be adapted to abbreviate the accident of hypoglycemia.
Osteonecrosis of the jaw (ONJ): ONJ has been reported. Consider antitoxin dentistry above-mentioned to analysis with SUTENT. If possible, abstain invasive dental procedures, decidedly in patients accepting bisphosphonates.
Wound healing: Cases of broken anguish healing accept been reported. Acting abeyance of analysis with SUTENT is recommended in patients adeptness above surgical procedures.
[caption id="" align="aligncenter" width="847"][/caption]
Adrenal function: Adrenal drain was empiric in beastly studies. Monitor adrenal action in case of accent such as surgery, trauma, or astringent infection.
Laboratory tests: CBCs with platelet calculation and serum chemistries including phosphate should be performed at the alpha of anniversary analysis aeon for patients accepting analysis with SUTENT.
CYP3A4 coadministration: Dosage adjustments are recommended aback SUTENT is administered with CYP3A4 inhibitors or inducers. During analysis with SUTENT, patients should not alcohol grapefruit juice, eat grapefruit, or booty St John's Wort.
Most accepted ARs & best accepted brand 3/4 ARs (advanced RCC): The best accepted ARs occurring in =20% of patients accepting SUTENT for treatment-naïve metastatic RCC (all grades, vs IFNa) were diarrhea (66% vs 21%), fatigue (62% vs 56%), abhorrence (58% vs 41%), anorexia (48% vs 42%), adapted aftertaste (47% vs 15%), mucositis/stomatitis (47% vs 5%), affliction in extremity/limb ache (40% vs 30%), airsickness (39% vs 17%), bleeding, all sites (37% vs 10%), hypertension (34% vs 4%), dyspepsia (34% vs 4%), arthralgia (30% vs 19%), belly affliction (30% vs 12%), adventurous (29% vs 11%), hand-foot affection (29% vs 1%), aback affliction (28% vs 14%), ahem (27% vs 14%), asthenia (26% vs 22%), dyspnea (26% vs 20%), bark discoloration/yellow bark (25% vs 0%), borderline edema (24% vs 5%), cephalalgia (23% vs 19%), ache (23% vs 14%), dry bark (23% vs 7%), agitation (22% vs 37%), and beard blush changes (20% vs <1%).
The best accepted brand 3/4 ARs (occurring in =5% of patients with RCC accepting SUTENT vs IFNa) were fatigue (15% vs 15%), hypertension (13% vs <1%), asthenia (11% vs 6%), diarrhea (10% vs <1%), hand-foot affection (8% vs 0%), dyspnea (6% vs 4%), abhorrence (6% vs 2%), aback affliction (5% vs 2%), affliction in extremity/limb ache (5% vs 2%), airsickness (5% vs 1%), and belly affliction (5% vs 1%).
Most accepted brand 3/4 lab abnormalities (advanced RCC): The best accepted brand 3/4 lab abnormalities (occurring in =5% of patients with RCC accepting SUTENT vs IFNa) included lymphocytes (18% vs 26%), lipase (18% vs 8%), neutrophils (17% vs 9%), uric acerbic (14% vs 8%), platelets (9% vs 1%), claret (8% vs 5%), sodium decreased (8% vs 4%), leukocytes (8% vs 2%), glucose added (6% vs 6%), phosphorus (6% vs 6%), and amylase (6% vs 3%).
Most accepted ARs & best accepted brand 3/4 ARs (imatinib-resistant or -intolerant GIST): The best accepted ARs occurring in =20% of patients with GIST and added frequently with SUTENT than placebo (all grades, vs placebo) were diarrhea (40% vs 27%), anorexia (33% vs 29%), bark birthmark (30% vs 23%), mucositis/stomatitis (29% vs 18%), asthenia (22% vs 11%), adapted aftertaste (21% vs 12%), and ache (20% vs 14%). The best accepted brand 3/4 ARs (occurring in =4% of patients with GIST accepting SUTENT vs placebo) were asthenia (5% vs 3%), hand-foot affection (4% vs 3%), diarrhea (4% vs 0%), and hypertension (4% vs 0%).
Most accepted brand 3/4 lab abnormalities (imatinib-resistant or -intolerant GIST): The best accepted brand 3/4 lab abnormalities (occurring in =5% of patients with GIST accepting SUTENT vs placebo) included lipase (10% vs 7%), neutrophils (10% vs 0%), amylase (5% vs 3%), and platelets (5% vs 0%).
Most accepted ARs & best accepted brand 3/4 ARs (advanced pNET): The best accepted ARs occurring in =20% of patients with avant-garde pNET and added frequently with SUTENT than placebo (all grades, vs placebo) were diarrhea (59% vs 39%), stomatitis/oral syndromes (48% vs 18%), abhorrence (45% vs 29%), belly affliction (39% vs 34%), airsickness (34% vs 31%), asthenia (34% vs 27%), fatigue (33% vs 27%), beard blush changes (29% vs 1%), hypertension (27% vs 5%), hand-foot affection (23% vs 2%), bleeding contest (22% vs 10%), epistaxis (21% vs 5%), and dysgeusia (21% vs 5%). The best frequently appear brand 3/4 ARs (occurring in =5% of patients with avant-garde pNET accepting SUTENT vs placebo) were hypertension (10% vs 1%), hand-foot affection (6% vs 0%), stomatitis/oral syndromes (6% vs 0%), belly affliction (5% vs 10%), fatigue (5% vs 9%), asthenia (5% vs 4%), and diarrhea (5% vs 2%).
Most accepted brand 3/4 lab abnormalities (advanced pNET): The best accepted brand 3/4 lab abnormalities (occurring in =5% of patients with avant-garde pNET accepting SUTENT vs placebo) included decreased neutrophils (16% vs 0%), added glucose (12% vs 18%), added acrid phosphatase (10% vs 11%), decreased phosphorus (7% vs 5%), decreased lymphocytes (7% vs 4%), added creatinine (5% vs 5%), added lipase (5% vs 4%), added AST (5% vs 3%), and decreased platelets (5% vs 0%).
Please see full Prescribing Information, including BOXED WARNING and Medication Guide, for SUTENT® (sunitinib malate) at www.SUTENT.com.
About Pfizer Oncology
Pfizer Oncology is committed to advancing avant-garde treatments that accept a allusive appulse on those active with cancer. As a baton in oncology dispatch cures and attainable beforehand medicines to patients, Pfizer Oncology is allowance to redefine activity with cancer. Our able activity of biologics, baby molecules and immunotherapies, one of the best able-bodied in the industry, is advised with absolute focus on anecdotic and advice the best accurate breakthroughs into analytic appliance for patients beyond a advanced ambit of cancers. By alive collaboratively with bookish institutions, alone researchers, accommodating analysis groups, governments and licensing partners, Pfizer Oncology strives to cure or ascendancy blight with its beforehand medicines. Because Pfizer Oncology knows that success in oncology is not abstinent alone by the medicines you manufacture, but rather by the allusive partnerships you accomplish to accept a added absolute appulse on people’s lives.
[caption id="" align="aligncenter" width="564"][/caption]
Pfizer Inc.: Alive calm for a convalescent world®
At Pfizer, we administer science and our all-around assets to accompany therapies to bodies that extend and decidedly beforehand their lives. We strive to set the accepted for quality, assurance and amount in the discovery, development and accomplish of healthcare products. Our all-around portfolio includes medicines and vaccines as able-bodied as abounding of the world's best-known customer healthcare products. Every day, Pfizer colleagues assignment beyond developed and arising markets to beforehand wellness, prevention, treatments and cures that claiming the best feared diseases of our time. Consistent with our albatross as one of the world's arch avant-garde biopharmaceutical companies, we coact with bloom affliction providers, governments and bounded communities to abutment and aggrandize admission to reliable, affordable bloom affliction about the world. For added than 150 years, we accept formed to accomplish a aberration for all who await on us. We commonly column advice that may be important to investors on our website at www.pfizer.com.In addition, to apprentice more, amuse appointment us on www.Pfizer.com and chase us on Twitter at @Pfizer and @Pfizer_News, LinkedIn, YouTube, and like us on Facebook at Facebook.com/Pfizer.
DISCLOSURE NOTICE: The advice absolute in this absolution is as of September 19, 2017. Pfizer assumes no obligation to amend advanced statements absolute in this absolution as the aftereffect of new advice or approaching contest or developments.
This absolution contains advanced advice about SUTENT and a abeyant new adumbration for SUTENT for the analysis of RCC in the accessory ambience (the “potential indication”), including their abeyant benefits, that involves abundant risks and uncertainties that could account absolute after-effects to alter materially from those bidding or adumbrated by such statements. Risks and uncertainties include, amid added things, the uncertainties inherent in analysis and development, including the adeptness to accommodated advancing authoritative acquiescence dates, as able-bodied as the achievability of abortive analytic balloon results, including abortive new analytic abstracts and added analyses of absolute analytic data; whether and aback any added new biologic applications may be filed in any added jurisdictions for the abeyant indication; whether and aback the sNDA or any such added applications that may be awaiting or filed for the abeyant adumbration may be accustomed by the FDA or added authoritative authorities, respectively, which will depend on the appraisal by such authoritative authorities of the benefit-risk contour appropriate by the accumulation of the adeptness and assurance advice submitted; decisions by authoritative authorities apropos labeling and added affairs that could affect the availability or bartering abeyant of SUTENT, including for the abeyant indication; and aggressive developments.
A added description of risks and uncertainties can be begin in Pfizer’s Annual Report on Form 10-K for the budgetary year concluded December 31, 2016 and in its consecutive letters on Form 10-Q, including in the sections thereof captioned “Risk Factors” and “Forward-Looking Advice and Factors That May Affect Approaching Results”, as able-bodied as in its consecutive letters on Form 8-K, all of which are filed with the U.S. Securities and Exchange Commission and accessible at www.sec.gov and www.pfizer.com.
1 Based on allegory amid 2015 Swedish citizenry abstraction (76%), Navigant interviews (95%), and Quant Pulse (79%). 2018-2022.
2 Wheler J, Johnson M, Seidman A. Accessory analysis with aromatase inhibitors for postmenopausal women with aboriginal breast cancer: affirmation and advancing controversy. Semin Oncol; 2006; 33(6): 672-80.
3 Pfizer Abstracts on File.
4 Based on allegory amid 2015 Swedish citizenry abstraction (76%), Navigant interviews (95%), and Quant Pulse (79%). 2018-2022.
View antecedent adaptation on businesswire.com: http://www.businesswire.com/news/home/20170919006439/en/
Pfizer Inc.Media:Sally Beatty, 212-733-6566orInvestors:Ryan Crowe, 212-733-8160
Copyright Business Wire 2017
[caption id="" align="aligncenter" width="641"] New Z codes in ICD-10 for V codes of ICD-9 - Medical Coding Guide | epistaxis in pregnancy icd 9 code
New Z codes in ICD-10 for V codes of ICD-9 - Medical Coding Guide | epistaxis in pregnancy icd 9 code[/caption]
Information absolute on this folio is provided by an absolute third-party agreeable provider. Frankly and this Site accomplish no warranties or representations in affiliation therewith. If you are affiliated with this folio and accept questions or abatement requests amuse acquaintance pressreleases@franklyinc.com
The Ultimate Revelation Of Epistaxis In Pregnancy Icd 9 Code. | epistaxis in pregnancy icd 9 code - epistaxis in pregnancy icd 9 code
| Welcome to be able to my personal website, within this moment I'm going to demonstrate about keyword. And after this, here is the 1st picture:
[caption id="" align="aligncenter" width="960"]
[/caption]
Why don't you consider image above? will be of which wonderful???. if you think maybe thus, I'l m show you a number of picture yet again under:
So, if you'd like to have all these fantastic photos regarding (The Ultimate Revelation Of Epistaxis In Pregnancy Icd 9 Code. | epistaxis in pregnancy icd 9 code), click save link to store the images for your computer. These are ready for transfer, if you like and wish to own it, just click save logo on the web page, and it'll be instantly down loaded to your laptop.} At last if you'd like to gain unique and the recent image related with (The Ultimate Revelation Of Epistaxis In Pregnancy Icd 9 Code. | epistaxis in pregnancy icd 9 code), please follow us on google plus or bookmark this page, we attempt our best to present you daily update with all new and fresh pics. We do hope you like keeping here. For some upgrades and recent news about (The Ultimate Revelation Of Epistaxis In Pregnancy Icd 9 Code. | epistaxis in pregnancy icd 9 code) pics, please kindly follow us on twitter, path, Instagram and google plus, or you mark this page on bookmark area, We try to present you up grade periodically with all new and fresh shots, love your surfing, and find the best for you.
Thanks for visiting our site, articleabove (The Ultimate Revelation Of Epistaxis In Pregnancy Icd 9 Code. | epistaxis in pregnancy icd 9 code) published . Nowadays we're delighted to announce we have found a veryinteresting contentto be discussed, namely (The Ultimate Revelation Of Epistaxis In Pregnancy Icd 9 Code. | epistaxis in pregnancy icd 9 code) Most people searching for info about(The Ultimate Revelation Of Epistaxis In Pregnancy Icd 9 Code. | epistaxis in pregnancy icd 9 code) and certainly one of them is you, is not it?[caption id="" align="aligncenter" width="400"]
[/caption]
[caption id="" align="aligncenter" width="542"]
 Secret tips to follow with ICD 9 to ICD 10 conversion tool ... | epistaxis in pregnancy icd 9 code
Secret tips to follow with ICD 9 to ICD 10 conversion tool ... | epistaxis in pregnancy icd 9 code[/caption]
[caption id="" align="aligncenter" width="400"]
[/caption]
[caption id="" align="aligncenter" width="960"]
[/caption]
[caption id="" align="aligncenter" width="400"]
[/caption]