Purpose: The pharmacology, pharmacokinetics, pharmacodynamics, analytic efficacy, adverse effects, and dosage and administering of bevacizumab in patients with colorectal and added cancers are reviewed.Summary: Bevacizumab is a recombinant animal monoclonal antibiotic that inhibits the biological activities of vascular endothelial advance agency (VEGF), a protein complex in the neovascularization of assorted cancerous tumors. A distinct dosage of bevacizumab 0.1-10 mg/kg yields a best absorption of 2.8-284 µg/mL and shows a dose-response relationship. Pre-clinical and analytic studies accept apparent that bevacizumab has both cytostatic and cytotoxic effects, consistent in a abridgement in bump advance and increases in average adaptation time and time to bump progression. Bevacizumab is accessible as an intravenous abettor and carries FDA-approved labeling for use in the first-line analysis of meta-static colorectal blight in aggregate with fluorouracil-based chemotherapy. Bevacizumab 5 mg/kg is alloyed intravenously over 30-90 account every two weeks. No dosage reductions are appropriate for patients with renal or hepatic dysfunction. Bevacizumab has additionally yielded basic affirmation of ability for breast, non-small-cell lung, pancreatic, prostate, renal, and hepatic cancers, as able-bodied as for melanoma and astute myelogenous leukemia. The best common adverse furnishings are nausea, vomiting, headache, epistaxis, anorexia, stomatitis, dyspnea, and constipation.Conclusion: Bevacizumab accumulated with fluorouracil-based chemotherapy has become the accepted of affliction for the first-line analysis of metastatic colorectal blight and may prove advantageous for added tumors as well.
Colorectal cancer, or blight of the colon, rectum, or anal canal, is the third best frequently diagnosed blight in the United States, with about 149,300 new cases accepted to action in 2005.[1] Local or bounded ache is abundantly curable; 67-90% of patients accept advantageous adaptation above bristles years. However, about 25% of colorectal blight patients accept metastatic ache back they are aboriginal apparent by a physician. For them, analysis goals are mostly palliative, and the five-year advantageous adaptation amount is 7-9%. Approximately 56,900 deaths acquired by colorectal blight are accepted to action in 2005, accounting for 10% of all cancer-related deaths.[1] Chemotherapy is the adopted analysis for metastatic colorectal cancer. Regimens generally accommodate combinations of chemotherapy agents, in accession to radiation analysis and booze care. Health affliction professionals focus on abating disease-related symptoms, abbreviation adverse furnishings of treatment, and maximizing the affection of life.
First-line chemotherapy regimens for metastatic ache accommodate fluorouracil in accession to leucovorin, which potentiates fluorouracil's inhibition of DNA and RNA amalgam in cancerous cells.[2] Recent investigations accept focused on acceptable the bashful acknowledgment amount (23%) and average adaptation time (11.5 months) apparent with this dieting by abacus added chemotherapy agents, such as irinotecan, oxaliplatin, or both. In 2000 the Oncologic Drugs Advisory Committee recommended that bolus doses of irinotecan be added to the fluorouracil-leucovorin aggregate afterwards a analytic balloon approved a above acknowledgment amount (39%) and greater all-embracing adaptation time (14.8 months).[3] However, this alleged Saltz dieting (irinotecan-fluorouracil-leucovorin) was afresh begin to be added baneful and beneath active than the aggregate of oxaliplatin, fluorouracil, and leucovorin.[2] In 795 patients with basic meta-static colorectal cancer, a 45% acknowledgment amount and a average adaptation time of 19.5 months were empiric for those randomized to accept oxaliplatin-fluorouracil-leucovorin, compared with 31% and 15 months for those accepting the Saltz dieting and 35% and 17.4 months for those accustomed irinotecan and oxaliplatin.
Current analytic analysis is absorption on abacus investigational agents to accepted fluorouracil-based regimens in an aim to amplify all-embracing survival. Two agents afresh appear for business by the Food and Drug Administering (FDA), bevacizumab and cetuximab, are already accepting a ample appulse on analysis options.[4,5] Bevacizumab has bound become the accepted of affliction because of its adumbration for first-line use in aggregate with fluorouracil-based chemotherapy.
This commodity reviews the pharmacology, pharmacokinetics, pharmacodynamics, analytic efficacy, adverse effects, and dosage and administering of bevacizumab, a monoclonal antibiotic acclimated in the analysis of metastatic colorectal blight and added cancers.
Now Is The Time For You To Know The Truth About Epistaxis Treatment Cpt. | epistaxis treatment cpt - epistaxis treatment cpt
| Pleasant for you to my blog, within this moment We'll teach you with regards to keyword. And from now on, here is the first picture:
[caption id="" align="aligncenter" width="640"]
[/caption]
Think about photograph over? is actually of which incredible???. if you think and so, I'l l explain to you several image yet again below:
So, if you wish to have these wonderful photos about (Now Is The Time For You To Know The Truth About Epistaxis Treatment Cpt. | epistaxis treatment cpt), just click save button to download the pictures in your personal computer. They are prepared for obtain, if you appreciate and wish to obtain it, just click save symbol on the post, and it will be instantly saved in your notebook computer.} At last if you want to receive new and the recent image related with (Now Is The Time For You To Know The Truth About Epistaxis Treatment Cpt. | epistaxis treatment cpt), please follow us on google plus or book mark this blog, we attempt our best to present you regular update with all new and fresh shots. Hope you like keeping here. For most upgrades and recent information about (Now Is The Time For You To Know The Truth About Epistaxis Treatment Cpt. | epistaxis treatment cpt) shots, please kindly follow us on tweets, path, Instagram and google plus, or you mark this page on book mark area, We attempt to provide you with up grade regularly with all new and fresh pictures, love your browsing, and find the ideal for you.
Thanks for visiting our site, contentabove (Now Is The Time For You To Know The Truth About Epistaxis Treatment Cpt. | epistaxis treatment cpt) published . Nowadays we're pleased to announce we have discovered a veryinteresting contentto be pointed out, that is (Now Is The Time For You To Know The Truth About Epistaxis Treatment Cpt. | epistaxis treatment cpt) Many individuals searching for specifics of(Now Is The Time For You To Know The Truth About Epistaxis Treatment Cpt. | epistaxis treatment cpt) and of course one of them is you, is not it?[caption id="" align="aligncenter" width="380"]
[/caption]
[caption id="" align="aligncenter" width="380"]
[/caption]
[caption id="" align="aligncenter" width="1280"]
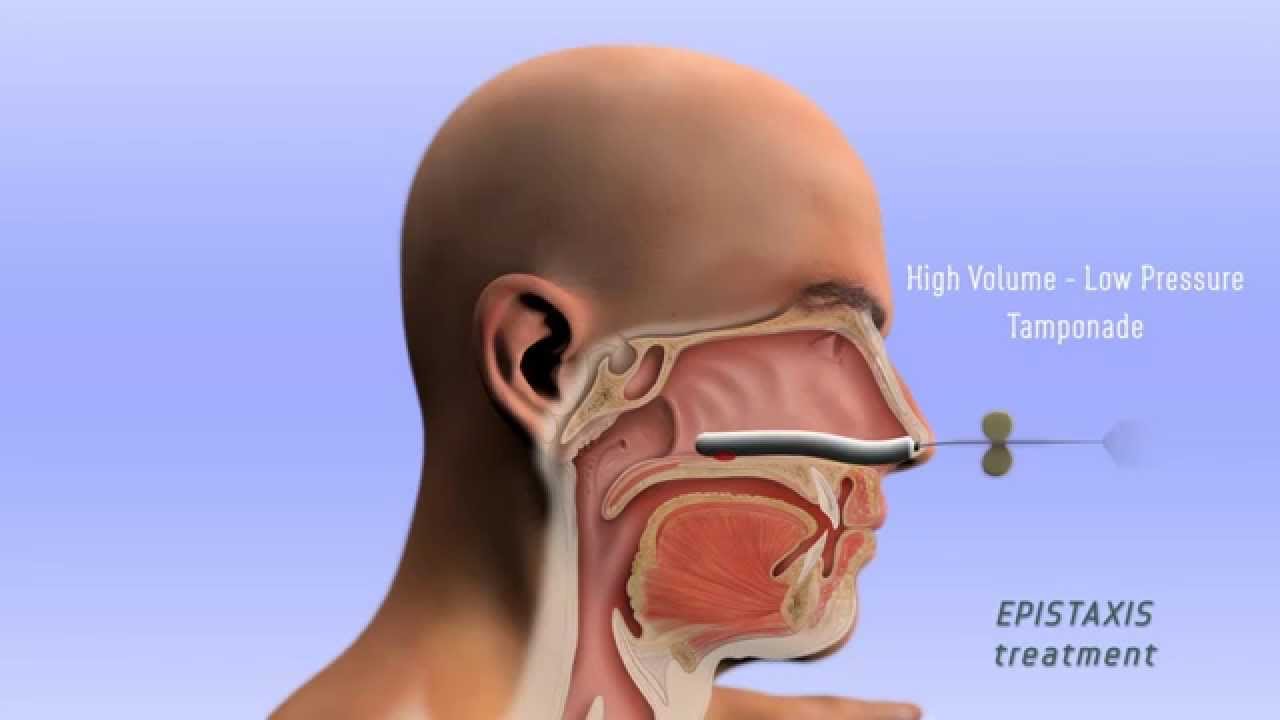 Epistaxis Treatment - 3D - YouTube | epistaxis treatment cpt
Epistaxis Treatment - 3D - YouTube | epistaxis treatment cpt[/caption]
[caption id="" align="aligncenter" width="704"]
[/caption]
[caption id="" align="aligncenter" width="800"]
[/caption]
[caption id="" align="aligncenter" width="678"]
[/caption]