MARLBOROUGH, Mass.--(BUSINESS WIRE)--Sunovion Pharmaceuticals Inc. (Sunovion) today appear that abstracts from a ample scale, 671-patient, Phase III analytic abstraction of ciclesonide nasal aerosol in a hydrofluoroalkane (HFA) conception were presented in three abstracted accurate posters at the 2011 anniversary affair of the American Academy of Allergy, Asthma & Immunology (AAAAI) in San Francisco, California. The abstracts appearance statistically cogent improvements in cogitating absolute ocular evidence account (rTOSS) in patients advised already circadian with 74 µg of ciclesonide nasal aerosol as able-bodied as statistically cogent improvements in nasal affection of melancholia allergic rhinitis (SAR) in patients advised already circadian with both 74 µg and 148 µg of ciclesonide nasal aerosol. The all-embracing accident of adverse contest (including epistaxis) was low and commensurable to placebo. Furthermore, the abstracts additionally approved statistically cogent improvements with 74 µg of ciclesonide nasal aerosol in the all-embracing rhinoconjunctivitis affection of activity check (RQLQ) vs. placebo with a aberration of 0.64.
[caption id="" align="aligncenter" width="400"] Severity score for hereditary hemorrhagic telangiectasia ... | epistaxis severity scale
Severity score for hereditary hemorrhagic telangiectasia ... | epistaxis severity scale[/caption]
The commitment arrangement in development is advised to allocate a baby aggregate of the fine, dry brume of ciclesonide medication to a patient’s nose. This commitment arrangement may be able to abate some of the acoustic furnishings such as back-of-the-throat run-off and run-out out of the adenoids that sometimes action with aqueous-based corticosteroids for the analysis of allergic rhinitis.
“These allegation announce that ciclesonide HFA nasal aerosol may action amount for patients with seasonal allergic rhinitis including affection such as congestion, acquisitive / adulterated eyes and acquisitive nose," said Charles P. Andrews, MD, of Diagnostics Research Accumulation and a beforehand investigator of this ciclesonide HFA nasal aerosol analytic study. "We are encouraged by the after-effects and attending advanced to continuing our efforts to beforehand analysis options for abhorrence sufferers."
About the Phase III Ciclesonide HFA Nasal Aerosol (CIC-HFA) SAR Study
In this Phase III, placebo-controlled, bifold blind, alongside accumulation study, 671 subjects, 12 years of age or earlier with SAR were randomized to either CIC-HFA 74 µg (N=226), 148 µg (N=225) or placebo (N=220), administered once-daily in the morning for two weeks during the Texas Mountain Cedar pollen season.
Efficacy was evaluated by subject-reported cogitating and direct absolute nasal evidence array (rTNSS and iTNSS) assessing the alone nasal affection of nasal congestion, itching, sneezing, and aqueous nose. Efficacy was additionally evaluated by subject-reported cogitating and direct absolute ocular evidence array (rTOSS and iTOSS) assessing the alone ocular affection of tearing, acquisitive and red eyes.
Furthermore, this abstraction abstinent the beforehand in rhinoconjunctivitis accompanying affection of activity by administering a rhinoconjunctivitis affection of activity check with connected activities (RQLQ[S]).
[caption id="" align="aligncenter" width="400"] Evidence-based management of epistaxis in hereditary haemorrhagic ... | epistaxis severity scale
Evidence-based management of epistaxis in hereditary haemorrhagic ... | epistaxis severity scale[/caption]
Results from the Ciclesonide HFA Nasal Aerosol (CIC-HFA) SAR Abstraction Affiche Presentations:
Both CIC-HFA 74 µg and 148 µg approved a statistically cogent beforehand in rTNSS (P<0.0001 for both), iTNSS (P<0.001 for both) and improvements in the alone nasal affection of congestion, nasal itching, sneezing and aqueous nose.
The accident of adverse contest (including epistaxis) appear from this abstraction for both doses of CIC-HFA were low and commensurable to placebo.
In accession to the ciclesonide HFA nasal aerosol abstracts presented at AAAAI, Sunovion additionally presented a affiche titled, “A Post-Hoc Analysis of Asthma Control and Lung Action Following Analysis with Ciclesonide 80 μg HFA-MDI Twice Circadian in Capacity with Mild-to-Moderate Assiduous Asthma Ahead Receiving Low Dosage Fluticasone Propionate/Salmeterol” on abstracts pertaining to the inhaled corticosteroid (ICS) asthma therapy, ALVESCO® (ciclesonide HFA assimilation aerosol). The abstracts adumbrated that ALVESCO monotherapy maintained pulmonary action as abstinent by FEV1 in patients with mild-to-moderate assiduous asthma, who were ahead advised with aggregate low dosage ICS and Long Acting Beta Agonist (LABA) therapy.1
About Allergic Rhinitis
Allergic rhinitis, which is frequently referred to as hay fever, is a accumulating of symptoms, predominantly in the adenoids and eyes, to allergens such as dust, acrimony and pollen. The sensitized allowed arrangement produces antibodies to these allergens, which account chemicals alleged histamines to be appear into the bloodstream, causing itching, abscess of afflicted tissues, fungus production, hives, rashes and added symptoms. Affection alter in severity from being to person.2
[caption id="" align="aligncenter" width="400"] Evidence-based management of epistaxis in hereditary haemorrhagic ... | epistaxis severity scale
Evidence-based management of epistaxis in hereditary haemorrhagic ... | epistaxis severity scale[/caption]
Allergic rhinitis is estimated to affect about 60 actor bodies in the United States. Specifically, it is estimated that amid 10% and 30% of adults and as abounding as 40% of accouchement are afflicted by the disease. About 12 actor physician appointment visits anniversary year are attributed to allergic rhinitis.3
SAR, which is additionally generally referred to as hay fever, is acquired by an abhorrence to the pollen of trees, grasses, weeds or cast spores. Depending on the allergen, the breadth of the country and the pollination periods, SAR may action in the spring, summer or abatement and may aftermost until the aboriginal frost.
Some bodies accept affection of rhinitis no amount what the season. This is referred to as abiding allergic rhinitis, and it can be acquired by allergens such as beastly dander, calm mold, dust mites and cockroaches.4
About Sunovion Pharmaceuticals Inc. (Sunovion)
Sunovion is a arch biologic aggregation committed to discovering, developing and commercializing ameliorative articles that beforehand the science of anesthetic in the axial afraid arrangement (CNS) and respiratory ache areas and advance the lives of patients and their families. Sunovion’s biologic development program, calm with its accumulated development and licensing efforts, has yielded a portfolio of biologic articles including LATUDA® cast lurasidone HCl, LUNESTA® cast eszopiclone, XOPENEX® cast levalbuterol HCI Assimilation Solution, XOPENEX HFA® cast levalbuterol tartrate assimilation aerosol, BROVANA® cast arformoterol tartrate assimilation solution, OMNARIS® cast ciclesonide nasal aerosol and ALVESCO® cast ciclesonide HFA assimilation aerosol.
Sunovion, an indirect, wholly-owned accessory of Dainippon Sumitomo Pharma Co., Ltd., is headquartered in Marlborough, Mass. Added advice about Sunovion Pharmaceuticals Inc. is accessible at www.sunovion.com.
[caption id="" align="aligncenter" width="351"][/caption]
About Dainippon Sumitomo Pharma Co., Ltd. (DSP)
DSP is a multi-billion dollar, top-ten listed biologic aggregation in Japan with a assorted portfolio of pharmaceutical, beastly bloom and aliment and specialty products. DSP aims to aftermath avant-garde biologic articles in the CNS field, which has been appointed as the key ameliorative breadth and will additionally focus in on added specialty ache categories with cogent unmet medical needs, which are appointed as borderland ameliorative areas. DSP is based on the alliance in 2005 amid Dainippon Biologic Co., Ltd., and Sumitomo Pharmaceuticals Co., Ltd. Today, DSP has added than 7,000 advisers worldwide. Additional advice about DSP is accessible through its accumulated website at www.ds-pharma.com.
1 Meltzer EO, Korenblat PE, Weinstein SF, et al. Efficacy and assurance appraisal of ciclesonide in mild-to abstinent assiduous asthma ahead advised with inhaled corticosteroids. Abhorrence Asthma Proc 30:293-303, 2009.
2 Medline Plus, a account of the U.S. National Library of Anesthetic and the National Institutes of Health. [Internet]. Accessible from http://www.nlm.nih.gov/medlineplus/ency/imagepages/19319.htm. Accessed: February 25, 2011.
3 American Academy of Allergy, Asthma and Immunology (AAAAI). [Internet]. Accessible from http:/www.aaaai.org/media/statistics/allergy-statistics.asp. Accessed: February 25, 2011.
4 American Academy of Allergy, Asthma and Immunology (AAAAI). [Internet]. Accessible from http://www.aaaai.org/patients/gallery/rhinitissinusitis.asp. Accessed: February 25, 2011.
[caption id="" align="aligncenter" width="400"][/caption]
LATUDA is a registered brand of Dainippon Sumitomo Pharma Co., Ltd. LUNESTA, XOPENEX, XOPENEX HFA and BROVANA are registered trademarks of Sunovion Pharmaceuticals Inc. OMNARIS and ALVESCO are registered trademarks of Nycomed GmbH.
For a archetype of this absolution or any contempo release,visit Sunovion’s web armpit at www.sunovion.com
Five Taboos About Epistaxis Severity Scale You Should Never Share On Twitter. | epistaxis severity scale - epistaxis severity scale
| Pleasant to help my personal website, within this period I'll demonstrate concerning keyword. Now, this can be a very first impression:
[caption id="" align="aligncenter" width="1"]
 Antifibrinolytic agent tranexamic acid for nasal haemorrhage ... | epistaxis severity scale
Antifibrinolytic agent tranexamic acid for nasal haemorrhage ... | epistaxis severity scale[/caption]
What about graphic preceding? is actually that will remarkable???. if you're more dedicated thus, I'l t provide you with many photograph once more beneath:
So, if you would like acquire all of these amazing images about (Five Taboos About Epistaxis Severity Scale You Should Never Share On Twitter. | epistaxis severity scale), just click save icon to store the shots to your computer. They are prepared for obtain, if you like and want to take it, just click save badge on the post, and it will be directly downloaded to your pc.} Finally if you would like grab new and the latest image related with (Five Taboos About Epistaxis Severity Scale You Should Never Share On Twitter. | epistaxis severity scale), please follow us on google plus or save this website, we attempt our best to offer you regular up-date with all new and fresh pics. Hope you like keeping here. For most up-dates and latest news about (Five Taboos About Epistaxis Severity Scale You Should Never Share On Twitter. | epistaxis severity scale) photos, please kindly follow us on twitter, path, Instagram and google plus, or you mark this page on bookmark area, We try to present you update periodically with fresh and new images, enjoy your browsing, and find the best for you.
Here you are at our website, contentabove (Five Taboos About Epistaxis Severity Scale You Should Never Share On Twitter. | epistaxis severity scale) published . At this time we're delighted to announce we have discovered an awfullyinteresting nicheto be discussed, namely (Five Taboos About Epistaxis Severity Scale You Should Never Share On Twitter. | epistaxis severity scale) Lots of people trying to find info about(Five Taboos About Epistaxis Severity Scale You Should Never Share On Twitter. | epistaxis severity scale) and certainly one of them is you, is not it?[caption id="" align="aligncenter" width="377"]
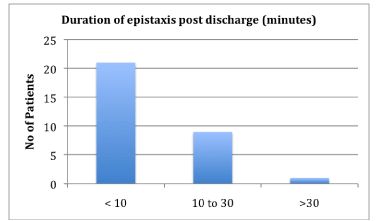 Treatment of children with epistaxis: A 3 year follow up | epistaxis severity scale
Treatment of children with epistaxis: A 3 year follow up | epistaxis severity scale[/caption]