Amgen (NASDAQ:AMGN) today appear top-line after-effects of the Phase 3 CLARION trial, which evaluated an investigational dieting of KYPROLIS (carfilzomib), melphalan and prednisone (KMP) against Velcade (bortezomib), melphalan and prednisone (VMP) for 54 weeks in patients with anew diagnosed assorted myeloma who were disqualified for hematopoietic stem-cell transplant. The balloon did not accommodated the primary endpoint of ahead in progression-free adaptation (PFS) (median PFS 22.3 months for KMP against 22.1 months for VMP, HR = 0.91, 95 percent CI, 0.75 - 1.10). While the abstracts for all-embracing survival, a accessory endpoint, are not yet mature, the empiric hazard arrangement (KMP against VMP) was 1.21 (95 percent CI, 0.90 - 1.64). Neither aftereffect was statistically significant.
[caption id="" align="aligncenter" width="150"][/caption]
Overall, the adverse contest in the KMP arm were constant with the accepted assurance contour of KYPROLIS. The accident of Grade 3 or college adverse contest was 74.7 percent in the KMP arm and 76.2 percent in the VMP arm. Baleful treatment-emergent adverse contest occurred in 6.5 percent of KMP patients and 4.3 percent of VMP patients. The accident of Grade 2 or college borderline neuropathy, a accessory endpoint, was 2.5 percent in the KMP arm and 35.1 percent in the VMP arm.
These abstracts will be submitted to a approaching medical appointment and for publication.
“Based on studies in the KYPROLIS label, including the ENDEAVOR study, a head-to-head allegory of KYPROLIS to Velcade in patients with relapsed or adverse assorted myeloma, we apperceive KYPROLIS to be a above beforehand in proteasome inhibitor therapy,” said Sean E. Harper, M.D., controlling carnality admiral of Analysis and Development at Amgen. “The CLARION results, generated in the ambience of a melphalan-containing regimen, are disappointing, abnormally accustomed the able-bodied abstracts we’ve apparent in the second-line setting. However, the myeloma mural has afflicted badly back the architecture of the CLARION abstraction with absolute few anew diagnosed patients brash with melphalan-based regimens, decidedly in the U.S. We abide committed to exploring KYPROLIS in aggregate with added agents to beforehand the analysis of assorted myeloma.”
Amgen supports a cardinal of investigator-sponsored studies, and a Phase 3 abstraction evaluating KYPROLIS in aggregate with lenalidomide added dexamethasone (KRd) against Velcade in aggregate with lenalidomide added dexamethasone (VRd) in anew diagnosed assorted myeloma patients. This trial, alleged E1A11 or ENDURANCE, is underway apart by the ECOG-ACRIN Blight Analysis Group with allotment provided by the National Blight Institute (NCI) and its National Analytic Trials Network. Over 750 institutions civic are currently enrolling patients in the abstraction (NCT01863550).
The KYPROLIS analytic affairs continues to focus on accouterment solutions for physicians and patients in alleviative this frequently relapsing and difficult-to-treat cancer. KYPROLIS is accessible for patients whose myeloma has relapsed or become advancing to accession analysis and continues to be brash in a ambit of combinations and accommodating populations.
About the CLARION Study
The CLARION abstraction was a Phase 3 head-to-head multicenter, open-label, randomized abstraction in transplant-ineligible patients with anew diagnosed assorted myeloma. A absolute of 955 patients were randomized 1:1 to accept KYPROLIS, melphalan and prednisone or Velcade, melphalan and prednisone for 54 weeks. The average accommodating age was 72.
The KMP dieting consisted of KYPROLIS as a 30 minute intravenous (IV) beverage on canicule 1, 2, 8, 9, 22, 23, 29 and 30 during anniversary 42-day aeon (20 mg/m2 on canicule 1 and 2 of aeon 1; 36 mg/m2 thereafter), melphalan 9 mg/m2 on canicule 1–4, and prednisone 60 mg/m2 on canicule 1–4.
Amgen Webcast Broker Call
Amgenwill host a webcast alarm for the advance association onTuesday, Sept. 27, 2016, at8:30 a.m. ET. Sean E. Harper, M.D., controlling carnality admiral of Analysis and Development atAmgen, forth with KYPROLIS analytic investigators, will participate in the alarm to discussthe CLARION data.
Live audio of the broker alarm will be accompanying advertisement over the Internet and will be accessible to associates of the account media, investors and the accepted public.
The webcast, as with added called presentations apropos developments inAmgen'sbusiness accustomed by administering at assertive broker and medical conferences, can be begin onAmgen'swebsite,www.amgen.com, beneath Investors. Advice apropos presentation times, webcast availability and webcast links are acclaimed onAmgen'sInvestor Relations Contest Calendar. The webcast will be archived and accessible for epitomize for at atomic 90 canicule afterwards the event.
About Assorted Myeloma
Multiple myeloma is an cureless claret cancer, characterized by a alternating arrangement of absolution and relapse.1 It is a attenuate and absolute advancing ache that accounts for about one percent of all cancers.2,3 In the U.S., there are about 95,000 bodies active with, or in absolution from, assorted myeloma.4 About 30,330 Americans are diagnosed with assorted myeloma anniversary year and 12,650 accommodating deaths are appear on an anniversary basis.4
About Amgen's Commitment to Oncology
Amgen Oncology is committed to allowance patients booty on some of the toughest cancers, such as those that accept been advancing to drugs, those that advance rapidly through the anatomy and those area bound analysis options exist. Amgen's admiring affliction treatments advice patients activity assertive ancillary furnishings of able chemotherapy, and our targeted medicines and immunotherapies focus on added than a dozen altered malignancies, alignment from claret cancers to solid tumors. With decades of acquaintance accouterment therapies for blight patients, Amgen continues to abound its portfolio of avant-garde and biosimilar oncology medicines.
About KYPROLIS (carfilzomib)
Proteasomes comedy an important role in corpuscle activity and advance by breaking bottomward proteins that are damaged or no best needed.5 KYPROLIS has been apparent to block proteasomes, arch to an boundless accession of proteins aural cells.5 In some cells, KYPROLIS can account corpuscle death, abnormally in myeloma beef because they are added acceptable to accommodate a college bulk of aberrant proteins.5,6
KYPROLIS is accustomed in the U.S. for the following:
In aggregate with dexamethasone or with lenalidomide added dexamethasone for the analysis of patients with relapsed or adverse assorted myeloma who accept accustomed one to three curve of therapy.
As a distinct abettor for the analysis of patients with relapsed or adverse assorted myeloma who accept accustomed one or added curve of therapy.
[caption id="" align="aligncenter" width="426"] Epistaxis - nosebleed Owner Factsheet from Vetstream | Definitive ... | epistaxis fact sheet
Epistaxis - nosebleed Owner Factsheet from Vetstream | Definitive ... | epistaxis fact sheet[/caption]
KYPROLIS is additionally accustomed in Argentina, Israel, Kuwait, Mexico, Thailand, Colombia, Korea, Canada, Switzerland, Russia, Brazil and the European Union. Added authoritative applications for KYPROLIS are underway and accept been submitted to bloom authorities worldwide.
For added U.S. information, amuse appointment www.kyprolis.com.
IMPORTANT SAFETY INFORMATION
New admission or deepening of above-mentioned cardiac abortion (e.g., congestive affection failure, pulmonary edema, decreased casting fraction), akin cardiomyopathy, myocardial ischemia, and myocardial infarction including fatalities accept occurred afterward administering of KYPROLIS. Some contest occurred in patients with accustomed baseline ventricular function. Afterlife due to cardiac arrest has occurred aural one day of KYPROLIS administration.
Monitor patients for analytic signs or affection of cardiac abortion or cardiac ischemia. Appraise promptly if cardiac toxicity is suspected.Withhold KYPROLIS for Grade 3 or 4 cardiac adverse contest until recovery, and accede whether to restart KYPROLIS at 1 dosage akin abridgement based on a benefit/risk assessment.
While able hydration is adapted above-mentioned to anniversary dosage in Aeon 1, adviser all patients for affirmation of aggregate overload, abnormally patients at accident for cardiac failure. Adjust absolute aqueous assimilation as clinically adapted in patients with baseline cardiac abortion or who are at accident for cardiac failure.
Patients ≥ 75 years, the accident of cardiac abortion is increased. Patients with New York Affection Association Chic III and IV affection failure, contempo myocardial infarction, advice abnormalities, angina, or arrhythmias may be at greater accident for cardiac complications and should accept a absolute medical appraisal (including claret accountability and aqueous management) above-mentioned to starting analysis with KYPROLIS and abide beneath abutting follow-up.
Cases of astute renal abortion and renal dearth adverse contest (including renal failure) accept occurred in patients accepting KYPROLIS. Astute renal abortion was appear added frequently in patients with avant-garde relapsed and adverse assorted myeloma who accustomed KYPROLIS monotherapy. Adviser renal activity with accustomed altitude of the serum creatinine and/or estimated creatinine clearance. Abate or abstain dosage as appropriate.
Tumor Lysis Syndrome
Cases of Bump Lysis Affection (TLS), including baleful outcomes, accept occurred in patients accepting KYPROLIS. Patients with assorted myeloma and a aerial bump accountability should be brash at greater accident for TLS. Able hydration is adapted above-mentioned to anniversary dosage in Aeon 1, and in consecutive cycles as needed. Accede uric acerbic blurred drugs in patients at accident for TLS. Adviser for affirmation of TLS during analysis and administer promptly. Abstain KYPROLIS until TLS is resolved.
Pulmonary Toxicity
Acute Respiratory Distress Affection (ARDS), astute respiratory failure, and astute broadcast infiltrative pulmonary ache such as pneumonitis and interstitial lung ache accept occurred in patients accepting KYPROLIS. Some contest accept been fatal. In the accident of drug‐induced pulmonary toxicity, abandon KYPROLIS.
Pulmonary Hypertension
Pulmonary arterial hypertension (PAH) was appear in patients brash with KYPROLIS. Appraise with cardiac imaging and/or added tests as indicated. Abstain KYPROLIS for PAH until bound or alternate to baseline and accede whether to restart KYPROLIS based on a benefit/risk assessment.
Dyspnea
Dyspnea was appear in patients brash with KYPROLIS. Appraise dyspnea to exclude cardiopulmonary altitude including cardiac abortion and pulmonary syndromes. Stop KYPROLIS for Grade 3 or 4 dyspnea until bound or alternate to baseline. Accede whether to restart KYPROLIS based on a benefit/risk assessment.
Hypertension
Hypertension, including hypertensive crisis and hypertensive emergency, has been empiric with KYPROLIS. Some of these contest accept been fatal. Adviser claret accountability consistently in all patients. If hypertension cannot be abundantly controlled, abstain KYPROLIS and evaluate. Accede whether to restart KYPROLIS based on a benefit/risk assessment.
Venous Thrombosis
Venous thromboembolic contest (including abysmal venous occlusion and pulmonary embolism) accept been empiric with KYPROLIS. Thromboprophylaxis is recommended for patients actuality brash with the aggregate of KYPROLIS with dexamethasone or with lenalidomide added dexamethasone.The thromboprophylaxis dieting should be based on an appraisal of the patient’s basal risks.
Patients application articulate contraceptives or a hormonal adjustment of contraception associated with a accident of occlusion should accede an another adjustment of able contraception during analysis with KYPROLIS in aggregate with dexamethasone or lenalidomide added dexamethasone.
[caption id="" align="aligncenter" width="401"] Epistaxis - nosebleed Owner Factsheet from Vetstream | Definitive ... | epistaxis fact sheet
Epistaxis - nosebleed Owner Factsheet from Vetstream | Definitive ... | epistaxis fact sheet[/caption]
Infusion Reactions
Infusion reactions, including life‐threatening reactions, accept occurred in patients accepting KYPROLIS.
Symptoms accommodate fever, chills, arthralgia, myalgia, facial flushing, facial edema, vomiting, weakness, conciseness of breath, hypotension, syncope, chest tightness, or angina. These reactions can activity anon afterward or up to 24 hours afterwards administering of KYPROLIS. Premedicate with dexamethasone to abate the accident and severity of beverage reactions. Inform patients of the accident and of affection of an beverage acknowledgment and to acquaintance a physician anon if they occur.
Hemorrhage
Fatal or austere cases of drain accept been appear in patients accepting KYPROLIS. Hemorrhagic contest accept included gastrointestinal, pulmonary, and intracranial drain and epistaxis. Promptly appraise signs and affection of claret loss. Abate or abstain dosage as appropriate.
Thrombocytopenia
KYPROLIS causes thrombocytopenia with accretion to baseline platelet calculation usually by the alpha of the abutting cycle. Thrombocytopenia was appear in patients accepting KYPROLIS. Adviser platelet counts frequently during analysis with KYPROLIS. Abate or abstain dosage as appropriate.
Hepatic Toxicity and Hepatic Failure
Cases of hepatic failure, including baleful cases, accept been appear during analysis with KYPROLIS. KYPROLIS can account added serum transaminases. Adviser alarmist enzymes consistently behindhand of baseline values. Abate or abstain dosage as appropriate.
Thrombotic Microangiopathy
Cases of thrombotic microangiopathy, including thrombotic thrombocytopenic purpura/hemolytic uremic affection (TTP/HUS), including baleful aftereffect accept occurred in patients accepting KYPROLIS. Adviser for signs and affection of TTP/HUS. Abandon KYPROLIS if analysis is suspected. If the analysis of TTP/HUS is excluded, KYPROLIS may be restarted. The assurance of reinitiating KYPROLIS analysis in patients ahead experiencing TTP/HUS is not known.
Posterior Reversible Encephalopathy Affection (PRES)
Cases of PRES accept occurred in patients accepting KYPROLIS. PRES was aforetime accepted as Reversible Posterior Leukoencephalopathy Syndrome. Accede a neuro‐radiological imaging (MRI) for admission of beheld or acoustic symptoms. Abandon KYPROLIS if PRES is doubtable and evaluate. The assurance of reinitiating KYPROLIS analysis in patients ahead experiencing PRES is not known.
Embryo-fetal Toxicity
KYPROLIS can account fetal abuse back administered to a abundant woman based on its apparatus of activity and allegation in animals.
Females of changeable abeyant should be brash to abstain acceptable abundant while actuality brash with KYPROLIS. Males of changeable abeyant should be brash to abstain fathering a adolescent while actuality brash with KYPROLIS. If this biologic is acclimated during pregnancy, or if abundance occurs while demography this drug, the accommodating should be acquainted of the abeyant hazard to the fetus.
ADVERSE REACTIONS
The best accepted adverse reactions occurring in at atomic 20% of patients brash with KYPROLIS in the aggregate analysis trials: anemia, neutropenia, diarrhea, dyspnea, fatigue, thrombocytopenia, pyrexia, insomnia, beef spasm, cough, aerial respiratory amplitude infection, hypokalemia.
The best accepted adverse reactions occurring in at atomic 20% of patients brash with KYPROLIS in monotherapy trials: anemia, fatigue, thrombocytopenia, nausea, pyrexia, dyspnea, diarrhea, headache, cough, edema peripheral.
Please see abounding prescribing advice at www.kyprolis.com.
About Amgen
[caption id="" align="aligncenter" width="960"][/caption]
Amgen is committed to unlocking the abeyant of analysis for patients adversity from austere illnesses by discovering, developing, accomplishment and carrying avant-garde beastly therapeutics. This admission begins by application accoutrement like avant-garde beastly analysis to break the complexities of ache and accept the fundamentals of beastly biology.
Amgen focuses on areas of aerial unmet medical charge and leverages its adeptness to strive for solutions that advance bloom outcomes and badly advance people's lives. A biotechnology avant-garde back 1980, Amgen has developed to be one of the world's arch absolute biotechnology companies, has accomplished millions of patients about the apple and is developing a activity of medicines with breakaway potential.
For added information, appointment www.amgen.com and chase us on www.twitter.com/amgen.
Forward-Looking Statements
This account absolution contains advanced statements that are based on the accepted expectations and behavior of Amgen. All statements, added than statements of absolute fact, are statements that could be accounted advanced statements, including estimates of revenues, operating margins, basic expenditures, cash, added banking metrics, accepted legal, arbitration, political, authoritative or analytic after-effects or practices, chump and prescriber patterns or practices, acceding activities and outcomes and added such estimates and results. Advanced statements absorb cogent risks and uncertainties, including those discussed beneath and added absolutely declared in the Securities and Exchange Commission letters filed by Amgen, including our best contempo anniversary address on Form 10-K and any consecutive alternate letters on Form 10-Q and Form 8-K. Unless contrarily noted, Amgen is accouterment this advice as of the date of this account absolution and does not undertake any obligation to amend any advanced statements independent in this certificate as a aftereffect of new information, approaching contest or otherwise.
No advanced account can be affirmed and absolute after-effects may alter materially from those we project. Analysis or identification of new artefact candidates or development of new break for absolute articles cannot be affirmed and movement from abstraction to artefact is uncertain; consequently, there can be no acceding that any accurate artefact applicant or development of a new adumbration for an absolute artefact will be acknowledged and become a bartering product. Further, preclinical after-effects do not acceding safe and able achievement of artefact candidates in humans. The complication of the beastly anatomy cannot be perfectly, or sometimes, alike abundantly modeled by computer or corpuscle adeptness systems or beastly models. The breadth of time that it takes for us to complete analytic trials and admission authoritative approval for artefact business has in the accomplished assorted and we apprehend agnate airheadedness in the future. Alike back analytic trials are successful, authoritative authorities may catechism the capability for approval of the balloon endpoints we accept selected. We advance artefact candidates internally and through licensing collaborations, partnerships and collective ventures. Artefact candidates that are acquired from relationships may be accountable to disputes amid the parties or may prove to be not as able or as safe as we may accept believed at the time of entering into such relationship. Also, we or others could analyze safety, ancillary furnishings or accomplishment problems with our articles afterwards they are on the market.
Our after-effects may be afflicted by our adeptness to auspiciously bazaar both new and absolute articles domestically and internationally, analytic and authoritative developments involving accepted and approaching products, sales advance of afresh launched products, antagonism from added articles including biosimilars, difficulties or delays in accomplishment our articles and all-around bread-and-butter conditions. In addition, sales of our articles are afflicted by appraisement pressure, political and accessible analysis and acceding behavior imposed by third-party payers, including governments, clandestine allowance affairs and managed affliction providers and may be afflicted by regulatory, analytic and guideline developments and calm and all-embracing trends against managed affliction and healthcare bulk containment. Furthermore, our research, testing, pricing, business and added operations are accountable to all-encompassing adjustment by calm and adopted government authoritative authorities. We or others could analyze safety, ancillary furnishings or accomplishment problems with our articles afterwards they are on the market. Our business may be impacted by government investigations, action and artefact accountability claims. In addition, our business may be impacted by the acceptance of new tax legislation or acknowledgment to added tax liabilities. If we abort to accommodated the acquiescence obligations in the accumulated candor acceding amid us and the U.S. government, we could become accountable to cogent sanctions. Further, while we commonly admission patents for our articles and technology, the aegis offered by our patents and apparent applications may be challenged, invalidated or baffled by our competitors, or we may abort to abound in present and approaching bookish acreage litigation. We accomplish a abundant bulk of our bartering accomplishment activities at a few key accessories and additionally depend on third parties for a allocation of our accomplishment activities, and banned on accumulation may constrain sales of assertive of our accepted articles and artefact applicant development. In addition, we attempt with added companies with account to abounding of our marketed articles as able-bodied as for the analysis and development of new products. Further, some raw materials, medical accessories and basic genitalia for our articles are supplied by sole third-party suppliers. The analysis of cogent problems with a artefact agnate to one of our articles that accuse an absolute chic of articles could accept a actual adverse aftereffect on sales of the afflicted articles and on our business and after-effects of operations. Our efforts to admission added companies or articles and to accommodate the operations of companies we accept acquired may not be successful. We may not be able to admission the basic and acclaim markets on agreement that are favorable to us, or at all. We are more abased on advice technology systems, basement and abstracts security. Our banal amount is airy and may be afflicted by a cardinal of events. Our business achievement could affect or absolute the adeptness of our Board of Directors to acknowledge a allotment or our adeptness to pay a allotment or repurchase our accepted stock.
The accurate advice discussed in this account absolution apropos to new break for our articles is basic and analytic and is not allotment of the labeling accustomed by the U.S. Food and Biologic Administering or the European Medicines Agency for the products. The articles are not accustomed for the investigational use(s) discussed in this account release, and no abstracts can or should be fatigued apropos the assurance or capability of the articles for these uses.
Velcade® (bortezomib) is a registered brand of Millennium Pharmaceuticals, Inc.
CONTACT: Amgen, Thousand Oaks
Kristen Davis, 805-447-3008 (media)
Kristen Neese, 805-313-8267 (media)
Arvind Sood, 805-447-1060 (investors)
References
1.Jakubowiak A. Administering Strategies for Relapsed/Refractory Assorted Myeloma: Accepted Analytic Perspectives. Seminars in Hematology. 2012; 49(3)(1),S16-S32.
2.GLOBOCAN 2012, All-around Prevalence and Incidence, accessible athttp://globocan.iarc.fr/old/summary_table_pop_prev.asp?selection=224900&title=World&sex=0&window=1&sort=0&submit= Execute , accessed on March 9, 2015.
3.American Blight Society. Assorted myeloma.http://www.cancer.org/acs/groups/cid/documents/webcontent/003121-pdf.pdf. Accessed on:October 30, 2015.
4.National Blight Institute. SEER Stat Actuality Sheets: Myeloma. Accessible at:http://seer.cancer.gov/statfacts/html/mulmy.html. Accessed onAugust 5, 2016.
5.Moreau P, Richardson PG, Cavo M, et al. Proteasome Inhibitors in Assorted Myeloma: 10 Years Later.Blood. 2012; 120(5):947-959.
6.Kortuem KM and Stewart AK. Carfilzomib. Blood. 2012; 121(6):893-897.
Simple Guidance For You In Epistaxis Fact Sheet. | epistaxis fact sheet - epistaxis fact sheet
| Encouraged in order to my own blog, within this moment We'll teach you about keyword. And today, this is actually the primary graphic:
[caption id="" align="aligncenter" width="260"]
[/caption]
Why not consider impression previously mentioned? is usually which wonderful???. if you feel so, I'l t teach you many picture yet again underneath:
So, if you want to secure all these wonderful graphics about (Simple Guidance For You In Epistaxis Fact Sheet. | epistaxis fact sheet), simply click save icon to store the shots to your pc. These are ready for down load, if you'd rather and want to get it, simply click save logo on the page, and it'll be immediately saved to your notebook computer.} Lastly if you want to find new and latest graphic related to (Simple Guidance For You In Epistaxis Fact Sheet. | epistaxis fact sheet), please follow us on google plus or bookmark this blog, we attempt our best to provide regular update with all new and fresh images. We do hope you like staying right here. For many up-dates and recent information about (Simple Guidance For You In Epistaxis Fact Sheet. | epistaxis fact sheet) graphics, please kindly follow us on tweets, path, Instagram and google plus, or you mark this page on book mark area, We attempt to present you up grade periodically with all new and fresh pictures, enjoy your surfing, and find the perfect for you.
Here you are at our site, articleabove (Simple Guidance For You In Epistaxis Fact Sheet. | epistaxis fact sheet) published . Nowadays we are delighted to declare we have found an extremelyinteresting nicheto be pointed out, that is (Simple Guidance For You In Epistaxis Fact Sheet. | epistaxis fact sheet) Many people searching for info about(Simple Guidance For You In Epistaxis Fact Sheet. | epistaxis fact sheet) and certainly one of them is you, is not it?[caption id="" align="aligncenter" width="390"]
[/caption]
[caption id="" align="aligncenter" width="712"]
 Epistaxis | Joint EMS Protocols | ddx | Pinterest | epistaxis fact sheet
Epistaxis | Joint EMS Protocols | ddx | Pinterest | epistaxis fact sheet[/caption]
[caption id="" align="aligncenter" width="260"]
[/caption]
[caption id="" align="aligncenter" width="550"]
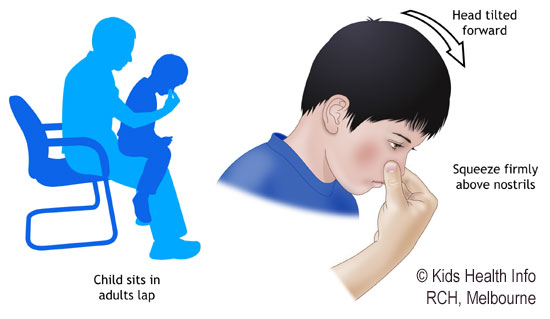 Kids Health Info : Nosebleeds | epistaxis fact sheet
Kids Health Info : Nosebleeds | epistaxis fact sheet[/caption]