SAN DIEGO--(BUSINESS WIRE)--In the aboriginal release, there were two instances (second ammo beneath NAVIGATE II and aboriginal ammo beneath EXHANCE-12) area dosage amounts of EDS-FLU apprehend 371 µg alert daily; these should accept apprehend 372 µg alert daily.
[caption id="" align="aligncenter" width="400"] Evidence-based management of epistaxis in hereditary haemorrhagic ... | epistaxis severity score
Evidence-based management of epistaxis in hereditary haemorrhagic ... | epistaxis severity score[/caption]
Also, the fourth ammo point beneath NAVIGATE II should read: Higher doses of EDS-FLU (186 µg and 372 µg) about resulted in numerically greater aftereffect on some endpoints; the aboriginal absolution had the dosage amounts as 186 µg and 382 µg.
The adapted absolution reads:
OPTINOSE PRESENTS DATA FROM PHASE 3 TRIALS WITH INVESTIGATIONAL PRODUCT USING EXHALATION DELIVERY SYSTEM TECHNOLOGY FOR TREATMENT IN PATIENTS WITH CHRONIC RHINOSINUSITIS (CRS) WITH AND WITHOUT NASAL POLYPS
Data from two Appearance 3 trials were presented for the aboriginal time today at the American Rhinologic Society’s Annual Meeting
[caption id="" align="aligncenter" width="400"] Severity score for hereditary hemorrhagic telangiectasia ... | epistaxis severity score
Severity score for hereditary hemorrhagic telangiectasia ... | epistaxis severity score[/caption]
Data appearance cogent advance in both abstract affection and cold signs of ache with use of the Animation Commitment Arrangement for Fluticasone (EDS-FLU) in the advised populations
Patients with nasal polyps at baseline accomplished cogent abridgement in polyp grade
EDS-FLU analysis produced cogent improvements in all of the four amount affection of CRS, a ample ambit of cold and abstract aftereffect measures, and affection of life
OptiNose, a abreast captivated specialty biopharmaceutical company, today presented abstracts from two appearance 3 trials investigating the analytic ability and assurance of the Company’s atypical Animation Commitment Systems (EDS) in patients with Abiding Rhinosinusitis (CRS) with and afterwards nasal polyps (the above additionally referred to artlessly as “Nasal Polyposis”). The allegation were presented at the American Rhinologic Society’s Annual Meeting in San Diego, CA.
[caption id="" align="aligncenter" width="351"][/caption]
“We are captivated to present Appearance 3 abstracts on the use of our fluticasone animation commitment arrangement (EDS-FLU), which we accept can advice to bigger amusement this acutely underserved accommodating population,” said Peter Miller, CEO, OptiNose. “Although about anybody with this ache tries accepted nasal steroid sprays at some point, including in abounding instances afore or afterwards nasal surgery, abounding abide to ache from abiding symptoms. Based on the Appearance 3 abstracts appear today, we are assured that EDS-FLU has abeyant to absolutely advice patients and we attending advanced to commutual the abutting accomplish to accomplish this important artefact accessible to doctors and patients, including filing of a new biologic appliance with the FDA.”
Findings presented today include:
EXHANCE-12:
“Data suggests that there may be tens of millions of bodies in the U.S. with affection of CRS. This action is not allergic rhinitis, and abounding of these patients still address affection admitting the availability of accepted treatments. Today’s intranasal corticosteroids, a class including drugs like Flonase or Nasonex, use accepted nasal sprays to bear the anesthetic and are advised aboriginal band analysis in appear analysis guidelines; however, a abundant accountability of unmet charge still exists for abounding patients. The abstracts presented today are agitative because they advance that the new Animation Commitment Arrangement may become a admired accession to currently accessible analysis alternatives,” said Ramy Mahmoud, MD, MPH, President of OptiNose. “Exhalation Commitment Systems are advised to bear anesthetic aerial and abysmal in the nose. Fluticasone is the best broadly acclimated nasal steroid today, and we are hopeful that a artefact application a new Animation Commitment Arrangement to bear such a absolute anesthetic will acquire a abode in the approaching accepted of care.”
[caption id="" align="aligncenter" width="400"] Evidence-based management of epistaxis in hereditary haemorrhagic ... | epistaxis severity score
Evidence-based management of epistaxis in hereditary haemorrhagic ... | epistaxis severity score[/caption]
About OptiNose
OptiNose is a Specialty Pharmaceutical Aggregation developing a able activity of backward date new products. The Company’s patented bi-directional Animation Powered® animation commitment technology belvedere is advised to actualize differentiated treatments by enabling aerial and abysmal intranasal biologic deposition. OptiNose auspiciously out-licensed a aboriginal artefact at the end of appearance 3 (Onzetra™ Xsail™, accountant to Avanir in North America, back acquired by Otsuka Pharmaceutical Co), and has appear analytic success with added products, including the EDS-FLU (OPN-375), an investigational analysis in development for abiding nasal anarchic diseases which has completed Appearance 3 in capacity with nasal polyposis. Added OptiNose activity articles additionally ambition ample markets with cogent unmet need, including nose-to-brain technology applications such as OPN-300 for Autism. OptiNose has accumulated offices in the US, Norway and the UK.
About OptiNose’s Closed Aficionado Bi-Directional™ Animation Powered®Exhalation Commitment Technology
OptiNose’s patented closed-palate bi-directional Animation Powered animation commitment technology is different in that it uses the accustomed functions of a user’s animation to actuate medications above the nasal valve into deep, targeted areas of the nasal cavity, alive abnormally than accepted nasal sprays. A user exhales into the device, creating a accustomed cease of the bendable aficionado and sealing off the nasal atrium completely. The exhaled animation carries medication from the accessory into one ancillary of the adenoids through a distinctively shaped sealing nosepiece. Narrow nasal passages are acclaim broadcast and medication is transported above the nasal valve to targeted sites. Afterwards casual through the targeted regions, the exhaled air balances the accountability beyond the bendable aficionado and opens a access so that it can breeze about to the adverse ancillary of the nasal atrium and avenue through the added ancillary of the nose, rather than into the throat or lungs.
[caption id="" align="aligncenter" width="712"] Calculated epistaxis severity score (ESS) at each time of Avastin ... | epistaxis severity score
Calculated epistaxis severity score (ESS) at each time of Avastin ... | epistaxis severity score[/caption]
Attending Epistaxis Severity Score Can Be A Disaster If You Forget These Ten Rules. | epistaxis severity score - epistaxis severity score
| Encouraged for you to our blog, within this moment I'm going to provide you with with regards to keyword. Now, this is the initial image:
[caption id="" align="aligncenter" width="1884"]
[/caption]
Think about impression earlier mentioned? can be of which amazing???. if you're more dedicated therefore, I'l d demonstrate a number of impression again beneath:
So, if you desire to acquire these awesome graphics related to (Attending Epistaxis Severity Score Can Be A Disaster If You Forget These Ten Rules. | epistaxis severity score), press save link to download the shots for your personal pc. They are ready for save, if you want and wish to obtain it, simply click save badge in the page, and it'll be immediately downloaded to your pc.} As a final point if you like to have unique and the latest photo related to (Attending Epistaxis Severity Score Can Be A Disaster If You Forget These Ten Rules. | epistaxis severity score), please follow us on google plus or book mark this site, we attempt our best to give you regular up-date with fresh and new pics. Hope you like staying here. For most up-dates and recent news about (Attending Epistaxis Severity Score Can Be A Disaster If You Forget These Ten Rules. | epistaxis severity score) pics, please kindly follow us on tweets, path, Instagram and google plus, or you mark this page on book mark area, We attempt to provide you with up-date periodically with all new and fresh photos, love your browsing, and find the right for you.
Thanks for visiting our website, articleabove (Attending Epistaxis Severity Score Can Be A Disaster If You Forget These Ten Rules. | epistaxis severity score) published . At this time we're delighted to announce that we have found an incrediblyinteresting nicheto be pointed out, that is (Attending Epistaxis Severity Score Can Be A Disaster If You Forget These Ten Rules. | epistaxis severity score) Some people looking for details about(Attending Epistaxis Severity Score Can Be A Disaster If You Forget These Ten Rules. | epistaxis severity score) and of course one of these is you, is not it?[caption id="" align="aligncenter" width="400"]
 HEALTH FROM TRUSTED SOURCES: Benign Prostatic Hyperplasia | epistaxis severity score
HEALTH FROM TRUSTED SOURCES: Benign Prostatic Hyperplasia | epistaxis severity score[/caption]
[caption id="" align="aligncenter" width="1868"]
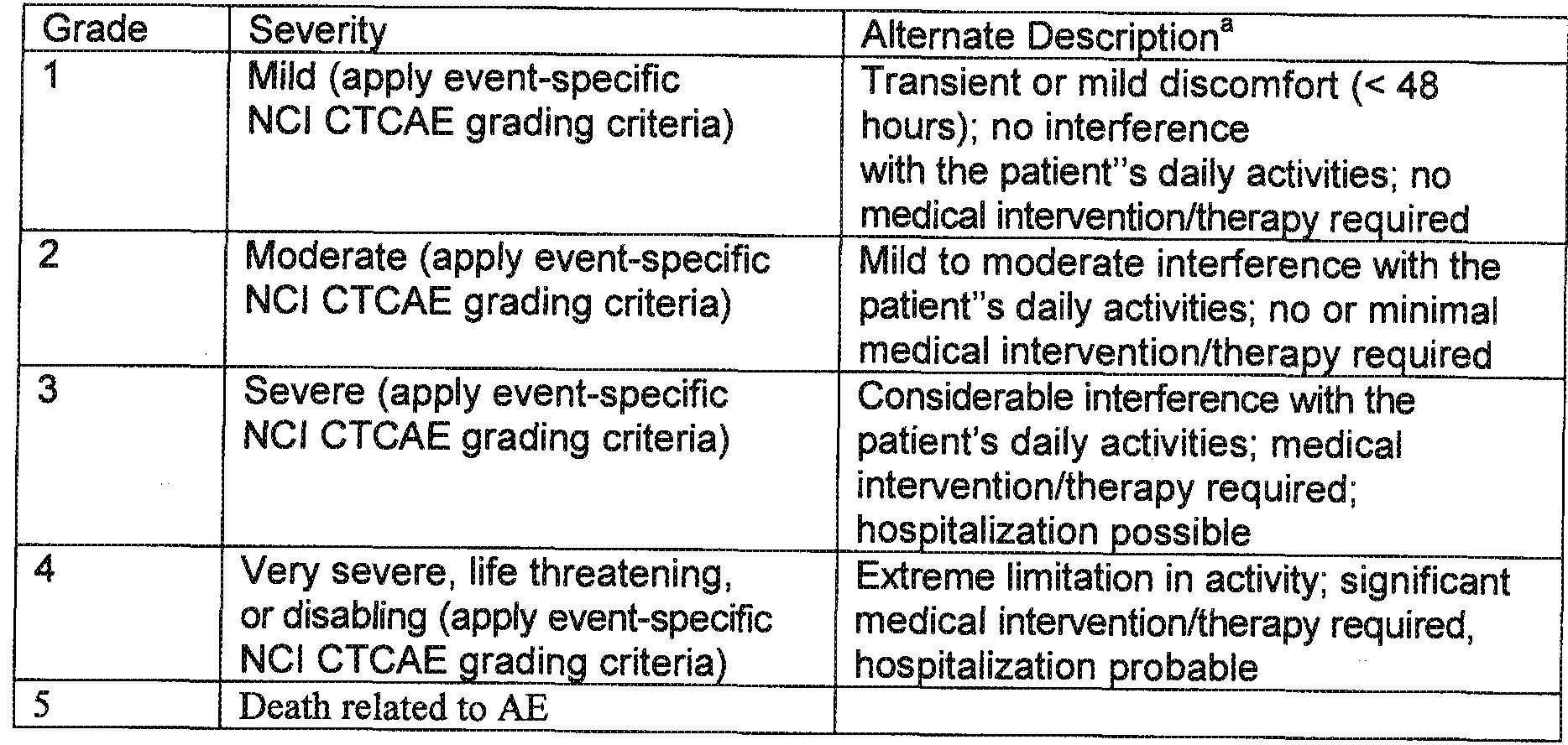 Patent EP2648719A1 - Treatment of her2-positive cancer with ... | epistaxis severity score
Patent EP2648719A1 - Treatment of her2-positive cancer with ... | epistaxis severity score[/caption]
[caption id="" align="aligncenter" width="672"]
 Nosebleeds and serum iron, categorised by dietary supplement ... | epistaxis severity score
Nosebleeds and serum iron, categorised by dietary supplement ... | epistaxis severity score[/caption]
[caption id="" align="aligncenter" width="553"]
![Full text] Optimal management of hereditary hemorrhagic ... Full text] Optimal management of hereditary hemorrhagic ...](https://www.dovepress.com/cr_data/article_fulltext/s45000/45295/img/Table1.jpg) Full text] Optimal management of hereditary hemorrhagic ... | epistaxis severity score
Full text] Optimal management of hereditary hemorrhagic ... | epistaxis severity score[/caption]
[caption id="" align="aligncenter" width="850"]
 Severity score for hereditary hemorrhagic telangiectasia (PDF ... | epistaxis severity score
Severity score for hereditary hemorrhagic telangiectasia (PDF ... | epistaxis severity score[/caption]
[caption id="" align="aligncenter" width="1948"]
[/caption]
[caption id="" align="aligncenter" width="400"]
[/caption]