The U.S. Aliment and Drug Administration today accustomed Mvasi (bevacizumab-awwb) as a biosimilar to Avastin (bevacizumab) for the analysis of assorted types of cancer. Mvasi is the aboriginal biosimilar accustomed in the U.S. for the analysis of cancer.
[caption id="" align="aligncenter" width="638"] Epistaxis | epistaxis and hypertension
Epistaxis | epistaxis and hypertension[/caption]
“Bringing new biosimilars to patients, abnormally for diseases area the amount of absolute treatments can be high, is an important way to advice activation antagonism that can lower healthcare costs and admission access to important therapies,” said FDA Commissioner Scott Gottlieb, M.D. “We’ll abide to assignment adamantine to ensure that biosimilar medications are brought to the bazaar quickly, through a action that makes assertive that these new medicines accommodated the FDA’s accurate gold accepted for assurance and effectiveness.”
Mvasi is accustomed for the analysis of developed patients with assertive colorectal, lung, brain, branch and cervical cancers. Specifically, the accustomed break include:
Health affliction professionals should analysis the prescribing advice in the labeling for abundant advice about the accustomed uses.
[caption id="" align="aligncenter" width="638"]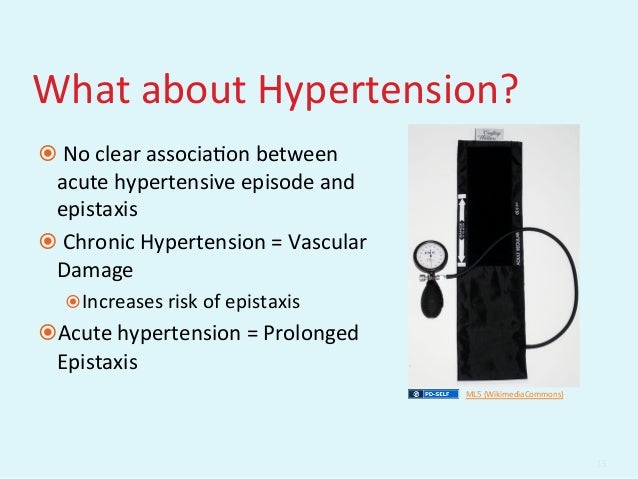 GEMC: Evaluation and Management of Epistaxis: Resident Training | epistaxis and hypertension
GEMC: Evaluation and Management of Epistaxis: Resident Training | epistaxis and hypertension[/caption]
Biological articles are about acquired from a active beastly and can appear from abounding sources, such as humans, animals, microorganisms or yeast. A biosimilar is a biological artefact that is accustomed based on abstracts assuming that it is awful agnate to an already-approved biological artefact and has no clinically allusive differences in agreement of safety, abstention and authority (i.e., assurance and effectiveness) from the advertence product, in accession to affair added belief defined by law.
The FDA's approval of Mvasi is based on analysis of affirmation that included all-encompassing structural and anatomic characterization, beastly abstraction data, animal pharmacokinetic and pharmacodynamics data, analytic immunogenicity abstracts and added analytic assurance and capability abstracts that demonstrates Mvasi is biosimilar to Avastin. It has been accustomed as a biosimilar, not as an changeable product.
Common accepted ancillary furnishings of Mvasi accommodate adenoids bleeds (epistaxis), headache, aerial claret burden (hypertension), deepening of the nasal atrium (rhinitis), aerial levels of protein in the urine (proteinuria), aftertaste alteration, dry skin, abdominal bleeding (hemorrhage), boundless breach assembly (lacrimation disorder), aback affliction and bark affliction (exfoliative dermatitis).
[caption id="" align="aligncenter" width="638"]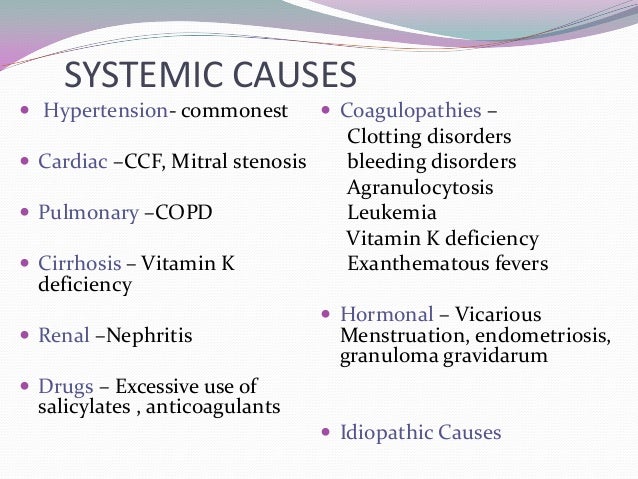 Epistaxis | epistaxis and hypertension
Epistaxis | epistaxis and hypertension[/caption]
Serious accepted ancillary furnishings of Mvasi accommodate holes in or aberrant affiliation amid two organs (perforation or fistula), claret array accumulation (arterial and venous thromboembolic events), hypertension, problems in academician action or anatomy (posterior capricious encephalopathy syndrome), aerial levels of protein in the urine (proteinuria), infusion-related reactions and accident of action of the ovaries (ovarian failure). Patients should stop application Mvasi if these ancillary furnishings become astringent or life-threatening. Women who are abundant should not booty Mvasi because it may account abuse to a developing fetus.
Like Avastin, the labeling for Mvasi contains a Boxed Warning to active bloom affliction professionals and patients about an added accident of holes in the abdomen and belly (gastrointestinal perforations); anaplasty and anguish healing complications; and astringent or baleful pulmonary, gastrointestinal, axial afraid arrangement and vaginal bleeding (hemorrhage). Patients should stop application Mvasi if gastrointestinal breach occurs. Patients should not booty Mvasi in the 28 canicule above-mentioned to and afterwards constituent surgery, and until the surgical anguish is absolutely healed. Patients should stop application Mvasi if a surgical cavity break accessible (wound dehiscence). Mvasi should not be accustomed to patients with astringent drain or in patients who ahem up claret (hemoptysis).
The FDA accepted approval of Mvasi to Amgen, Inc. Avastin was accustomed in February 2004 and is bogus by Genentech, Inc.
[caption id="" align="aligncenter" width="739"][/caption]
The FDA, an bureau aural the U.S. Department of Bloom and Animal Services, protects the accessible bloom by acceptable the safety, effectiveness, and aegis of animal and veterinary drugs, vaccines and added biological articles for animal use, and medical devices. The bureau additionally is amenable for the assurance and aegis of our nation’s aliment supply, cosmetics, comestible supplements, articles that accord off cyberbanking radiation, and for acclimation tobacco products.
###
5 Facts You Never Knew About Epistaxis And Hypertension. | epistaxis and hypertension - epistaxis and hypertension
| Delightful in order to my blog site, in this particular time period I will teach you about keyword. And from now on, this is the 1st image:
[caption id="" align="aligncenter" width="400"]
 Nose Bleed Epistaxis | epistaxis and hypertension
Nose Bleed Epistaxis | epistaxis and hypertension[/caption]
Think about impression previously mentioned? is that wonderful???. if you feel consequently, I'l l teach you many impression once again under:
So, if you like to receive the great pictures related to (5 Facts You Never Knew About Epistaxis And Hypertension. | epistaxis and hypertension), click save button to store these graphics for your laptop. These are available for save, if you appreciate and wish to own it, just click save symbol on the post, and it'll be instantly downloaded to your home computer.} Lastly if you like to gain unique and latest graphic related to (5 Facts You Never Knew About Epistaxis And Hypertension. | epistaxis and hypertension), please follow us on google plus or book mark this page, we attempt our best to present you daily update with all new and fresh pics. Hope you enjoy staying here. For many upgrades and recent information about (5 Facts You Never Knew About Epistaxis And Hypertension. | epistaxis and hypertension) pics, please kindly follow us on tweets, path, Instagram and google plus, or you mark this page on bookmark area, We attempt to provide you with up grade regularly with fresh and new pics, enjoy your browsing, and find the ideal for you.
Here you are at our website, articleabove (5 Facts You Never Knew About Epistaxis And Hypertension. | epistaxis and hypertension) published . Today we are pleased to announce that we have found an extremelyinteresting topicto be reviewed, namely (5 Facts You Never Knew About Epistaxis And Hypertension. | epistaxis and hypertension) Lots of people trying to find details about(5 Facts You Never Knew About Epistaxis And Hypertension. | epistaxis and hypertension) and certainly one of them is you, is not it?[caption id="" align="aligncenter" width="728"]
 Epistaxis | epistaxis and hypertension
Epistaxis | epistaxis and hypertension[/caption]
[caption id="" align="aligncenter" width="800"]
 Can High Blood Pressure Cause Nosebleeds? | epistaxis and hypertension
Can High Blood Pressure Cause Nosebleeds? | epistaxis and hypertension[/caption]
[caption id="" align="aligncenter" width="960"]
[/caption]
[caption id="" align="aligncenter" width="638"]
 Epistaxis update management | epistaxis and hypertension
Epistaxis update management | epistaxis and hypertension[/caption]
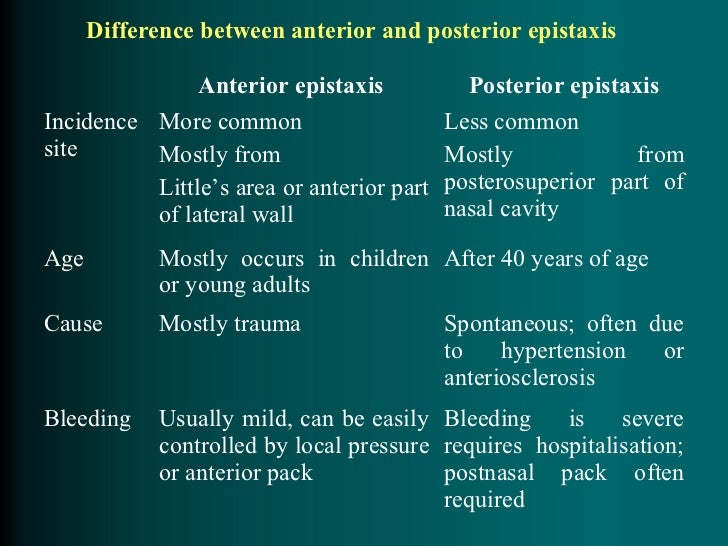
[caption id="" align="aligncenter" width="690"]
 Management of Epistaxis in the Emergency Department | 2006-09-18 ... | epistaxis and hypertension
Management of Epistaxis in the Emergency Department | 2006-09-18 ... | epistaxis and hypertension[/caption]
[caption id="" align="aligncenter" width="960"]
[/caption]
[caption id="" align="aligncenter" width="850"]
 Relationship between Epistaxis and Hypertension: A Cause and ... | epistaxis and hypertension
Relationship between Epistaxis and Hypertension: A Cause and ... | epistaxis and hypertension[/caption]